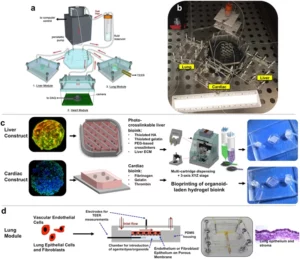
3D breast cancer models are rapidly redefining how we investigate the world’s deadliest malignancy in women, exposing biological complexities that flat monolayers and animal surrogates routinely overlook (For a historical lens on how innovations in therapeutic strategy emerged, see our companion piece, “Breast cancer organoid: A short history of innovation in breast cancer treatment.”). Despite the arrival of molecularly targeted agents, antibody–drug conjugates, and immunotherapies, breast cancer still accounts for more cancer‑related deaths among women than any other tumour type. Two major hurdles underlie this paradox: pronounced inter‑ and intratumoral heterogeneity, and the scarcity of experimental systems that faithfully reproduce the tumour microenvironment (TME) governing pathogenesis and treatment response.
Historically, xenografts and genetically engineered mouse models illuminated key hallmarks of malignancy, yet species‑specific differences, ethical constraints, and high costs blunt their translational impact (issues explored in “Breast cancer organoids as alternative to animal models.”). Two‑dimensional (2D) cultures of immortalised breast‑cancer cell lines, meanwhile, have fuelled decades of high‑throughput screening and pathway discovery but distort cell‑to‑cell and cell‑to‑matrix interactions and erase critical oxygen, nutrient, and drug‑diffusion gradients.
The emergence of patient‑derived organoids (PDOs) and other advanced 3D systems addresses these deficits head‑on. These self‑organising constructs conserve native cyto‑architecture, genomic diversity, and stromal cues, offering an experimentally tractable platform for precision oncology. In the pages that follow, we first contrast 2D monolayers with 3D breast cancer models to reveal hidden drug resistance, then examine how organoids reconstruct the TME and leverage microfluidic organ‑on‑chip technology for dynamic, immune‑competent tumour modeling. Together, these innovations chart a path toward more predictive pre‑clinical pipelines and ultimately more effective, personalised therapies for patients with breast cancer.
Learn more about our ready to use breast cancer organoid models.
2D Breast‑Cancer Cell Lines: Utility and Inherent Constraints
A vast panel of immortalised breast‑cancer cell lines anchors contemporary in vitro research. Pioneering work by Neve et al. classified 51 lines into luminal A, luminal B, HER2‑enriched and basal‑like (basal A and basal B) subsets that mirror the molecular taxonomies seen in tumours, providing investigators with a ready surrogate for each clinical subtype(1). Subsequent profiling efforts have expanded this catalogue to hundreds of lines annotated for mutations, copy‑number variation and drug response, enabling rational model selection for pathway analysis or high‑throughput screening.
Functional applications
Because the majority of well-characterized oncogenic drivers (PIK3CA, TP53, ESR1, ERBB2, BRCA1/2) are retained, 2D cultures remain indispensable for deciphering oncogenic signalling, synthetic‑lethal dependencies and endocrine‑resistance mechanisms, particularly in luminal models such as MCF‑7 or T‑47D(2). Their ease of propagation, genetic tractability and compatibility with robotic platforms have made them the cornerstone of compound libraries and CRISPR screens, accelerating the discovery of combination therapies across thousands of drug–gene interactions(3). Moreover, many lines form cell‑line–derived xenografts (CDX), offering an economical in‑vivo extension for pharmacodynamic or imaging studies before advancing to patient‑derived xenografts (PDX)(4).
Systemic limitations
Yet the very properties that make cell lines convenient impose biological limitations. Continuous passaging fosters genetic drift: independent MCF‑7 stocks, for example, diverge markedly in karyotype, growth rate and drug susceptibility after repeated freeze–thaw cycles or sub‑cloning, undermining reproducibility(5). Standard 2D plasticware further enforces a flattened morphology that disrupts apico‑basal polarity, homogenises nutrient and oxygen exposure, and disrupts stromal‑cell and extracellular‑matrix (ECM) contacts; as a consequence, transcriptomic and metabolic profiles deviate from those of primary tumours and dampen context‑dependent drug responses(6).
Compounding these issues, most index lines derive from late‑stage pleural effusions, so CDXs are ill‑suited for probing early oncogenic events or the emergence of endocrine resistance typical of hormone‑sensitive disease(7). Consequently, while CDXs remain indispensable for rapid, low‑cost pharmacodynamic studies, their interpretative scope must be confined to late‑stage, epithelial‑centric questions rather than the full spectrum of breast‑cancer evolution.
CDX‑specific concerns
When transplanted into immunodeficient mice, CDX tumours do recapitulate parental‑line genetics, yet they propagate as uniform epithelial masses devoid of stromal, vascular and immune elements that shape drug penetration and metastatic niche formation(4).
Compounding these issues, most index lines derive from late‑stage pleural effusions, so CDXs are ill‑suited for probing early oncogenic events or the emergence of endocrine resistance typical of hormone‑sensitive disease(7). Continuous in vitro passaging before engraftment drives genetic drift and clonal selection, producing divergent subclones with variable drug sensitivities that erode reproducibility and further narrow the biological heterogeneity originally present in patient tumours. Consequently, while CDXs remain indispensable for rapid, low‑cost pharmacodynamic studies, their interpretative scope must be confined to late‑stage, epithelial‑centric questions rather than the full spectrum of breast‑cancer evolution.
Implications for next-generation models
Together, these constraints underscore why 2D systems, though irreplaceable for mechanistic dissection, provide an incomplete lens on breast‑cancer biology. The advent of spheroids, scaffold‑based 3D cultures and patient‑derived organoids seeks to restore ECM architecture, preserve clonal diversity and enable co‑culture with immune or stromal partners ; capabilities discussed in the following section as we transition from traditional monolayers to 3D breast‑cancer models.
3D Breast Cancer Models: Drug Resistance, Microenvironment, and Dynamic Platforms
Three‑dimensional (3D) culture technologies are reshaping breast‑cancer research and organoid research by bridging the gap between oversimplified 2D monolayers and the complex in vivo tumour milieu characteristic of advanced 3D breast cancer models, a contrast at the core of ongoing 2D vs 3D cell culture debates. The overview below examines how 3D breast‑cancer models, including patient‑derived breast cancer organoids, reveal hidden drug resistance, reconstruct the tumour microenvironment, and harness dynamic organ‑on‑chip platforms for translational insight in contemporary tumor modeling.
3D breast cancer models expose hidden drug resistance
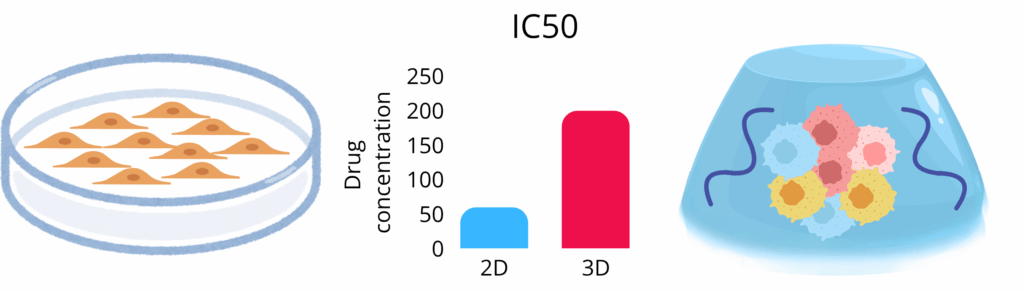
The architectural leap from flat monolayer to three‑dimensional (3D) culture fundamentally re‑programmes how breast‑cancer cells perceive and respond to therapy. A series of comparative studies underscores the magnitude of this shift. Imamura and colleagues demonstrated that luminal (T‑47D, BT‑474) and triple‑negative (BT‑549) lines grown as dense multicellular spheroids were markedly less susceptible to paclitaxel or doxorubicin than their two‑dimensional (2D) counterparts, mirroring the dormancy, hypoxia and anti‑apoptotic signalling encountered in vivo(8). Muguruma et al. extended these observations across thirteen triple‑negative breast‑cancer (TNBC) lines, reporting two‑ to ten‑fold increases in IC₅₀ values for epirubicin, cisplatin and docetaxel in 3D vs 2D cell culture (scaffold‑free spheroids); intriguingly, round compact spheroids were even more drug‑refractory than grape‑like aggregates, pointing to micro‑architecture as a determinant of chemoresistance(9). Complementary work from Stock et al. revealed that trastuzumab efficacy against HER2‑positive cells declines sharply under 3D conditions, coinciding with altered receptor clustering and downstream signalling dynamics(10).
These discordant drug responses arise because 3D breast‑cancer models simultaneously address two long‑standing limitations of conventional assays. Extrinsically, the use of extracellular‑matrix surrogates, whether basement‑membrane extract or synthetic hydrogels, reinstates gradient‑driven hypoxia, integrin‑mediated mechanotransduction and paracrine crosstalk (microenvironmental variables that modulate proliferation and apoptotic thresholds). Intrinsically, patient‑derived breast cancer organoids (PDOs) conserve the genomic, transcriptomic and histological complexity of their primary tumours far more faithfully than established lines. Recent organoid research led by Sachs et al. showed that over one hundred breast‑cancer organoid lines retained copy‑number landscapes and receptor phenotypes over twenty passages(11), while Chen et al. demonstrated that pharmaco‑phenotypes of PDOs from drug‑resistant patients predicted subsequent clinical responses(12). At the single‑cell level, Saeki et al. uncovered three to six transcriptionally distinct cancer‑cell states within individual organoid lines, confirming that intratumoral heterogeneity (including rare epithelial‑to‑mesenchymal intermediates) is preserved ex vivo(13).
Together, these findings position 3D breast‑cancer models as a more stringent and clinically resonant platform for pre‑clinical drug discovery: they embed tumour cells in a context that replicates both the extrinsic pressures of the tumour microenvironment and the intrinsic diversity that drives treatment failure.
Rebuilding the tumor microenvironment in 3D systems
A defining virtue of 3D breast cancer models for advanced tumor modeling is their capacity to restore the extracellular matrix (ECM) cues that govern both normal mammary function and malignant progression. In the healthy gland, a bilayered epithelium of luminal and basal cells branches into terminal ductal–lobular units whose lactational capacity depends on a compliant, laminin‑rich basement membrane embedded within adipose‑fibroblast stroma. Classic morphogenesis studies showed that alveolar differentiation and milk ejection require the correct spatial distribution of ECM components and biomechanical tension, features that 2D monolayers cannot reproduce. These differences underscore the limitations highlighted in 2D vs 3D cell culture comparisons. Recent tissue‑engineering reviews reiterate that this three‑dimensional architecture, together with hormonal and paracrine signalling, is indispensable for modelling breast biology in vitro(14).
Tumorigenesis rewires this matrix in both composition and mechanics. Hallmark alterations include loss of polarity‑sustaining laminin isoforms, up‑regulation of matrix metalloproteinases (MMPs), and pathological deposition of fibrillar collagens that cross‑link and stiffen the stroma. Normal breast tissue exhibits an elastic modulus of ~0.2 kPa, whereas invasive lesions exceed 4 kPa, a biophysical shift that accelerates epithelial–mesenchymal transition (EMT), activates PI3K signalling, and predicts metastatic risk. This phenomenon is responsible for tissue stiffness that serves as a diagnostic marker(14,15).
Advanced 3D platforms can now embed cancer cells within biomimetic matrices that mirror these disease‑specific cues. In an elegant proof‑of‑concept, Jin et al. decellularised matched normal and tumour biopsies to generate human breast ECM scaffolds. When reseeded with MCF‑7 cells, tumour‑derived scaffolds induced EMT and proliferative expansion, whereas normal scaffolds promoted apoptosis; direct evidence that biochemical and mechanical properties of the ECM dictate cell fate(16). Such findings underscore why integrating native‑like matrices or tunable hydrogels into organoid culture is critical for capturing drug resistance linked to stromal remodelling.
To learn more on how ECM properties affect tumour and how PlastCell, our EU PathFinder project, is creating a microfluidic imaging platform that tracks how live cancer cells adapt to stress, quantifying plasticity and predicting tumour aggressiveness visit our wepbage.
Beyond the matrix, patient‑derived breast cancer organoids (PDOs) can retain endogenous immune and stromal elements when cultured in air–liquid or microfluidic formats. Neal et al. demonstrated that PDOs established with an air–liquid interface preserve tumour‑infiltrating lymphocytes (TILs) and maintain their T‑cell receptor repertoire, enabling in‑vitro interrogation of PD‑1/PD‑L1 blockade within an intact tumour‑immune ecosystem(17). Coupling such immunocompetent PDOs with ECM‑engineered scaffolds therefore offers an unprecedented avenue to study how microenvironmental mechanics, soluble factors, and immune checkpoints interact to shape therapeutic response in breast cancer.
Microfluidic organ-on-chip: Dynamic & immune-competent 3D platforms
Microfluidic organ‑on‑chip (OoC) technology extends the advantages of static organoids highlighted in recent organoid research, pushing the frontier of tumor modeling, and by adding continual perfusion, controllable shear stress, and compartmentalized co‑culture. Breast‑cancer metastasis chips recreate endothelial barriers, chemokine gradients, and tissue‑specific ECM to track real‑time invasion and circulating‑tumour‑cell arrest, while permitting tumour‑infiltrating lymphocyte (TIL) analysis under defined oxygen tension. Dynamic flow also accelerates organoid growth and preserves drug‑response fidelity, trimming culture timelines from weeks to days without compromising histo‑molecular. Recent tumour‑on‑chip prototypes integrate electrospun scaffolds and dual‑channel perfusion to model stromal cross‑talk and deliver pharmacokinetics‑relevant dosing for high‑content screening(18–20).
Experience our human immunocompetent breast‑cancer organoid model: a perfused, vascularised tumoroid cultured on the CubiX™ microphysiological platform. By integrating patient‑derived tumour cells with CAF, immune and endothelial compartments in a tunable ECM scaffold, CubiX delivers human‑relevant data to accelerate your drug discovery. Explore our solution here.
Resources
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. déc 2006;10(6):515‑27.
- Dai X, Cheng H, Bai Z, Li J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J Cancer. 2017;8(16):3131‑41.
- Vis DJ, Jaaks P, Aben N, Coker EA, Barthorpe S, Beck A, et al. A pan-cancer screen identifies drug combination benefit in cancer cell lines at the individual and population level. Cell Rep Med. août 2024;5(8):101687.
- Souto EP, Dobrolecki LE, Villanueva H, Sikora AG, Lewis MT. In Vivo Modeling of Human Breast Cancer Using Cell Line and Patient-Derived Xenografts. J Mammary Gland Biol Neoplasia. juin 2022;27(2):211‑30.
- Ben-David U, Siranosian B, Ha G, Tang H, Oren Y, Hinohara K, et al. Genetic and transcriptional evolution alters cancer cell line drug response. Nature. août 2018;560(7718):325‑30.
- Mangani S, Kremmydas S, Karamanos NK. Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers. 30 mars 2025;17(7):1161.
- Ciringione A, Rizzi F. Facing the Challenge to Mimic Breast Cancer Heterogeneity: Established and Emerging Experimental Preclinical Models Integrated with Omics Technologies. Int J Mol Sci. 10 mai 2025;26(10):4572.
- Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep. avr 2015;33(4):1837‑43.
- Muguruma M, Teraoka S, Miyahara K, Ueda A, Asaoka M, Okazaki M, et al. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem Biophys Res Commun. déc 2020;533(3):268‑74.
- Stock K, Estrada MF, Vidic S, Gjerde K, Rudisch A, Santo VE, et al. Capturing tumor complexity in vitro: Comparative analysis of 2D and 3D tumor models for drug discovery. Sci Rep [Internet]. 1 juill 2016 [cité 17 juill 2025];6(1). Disponible sur: https://www.nature.com/articles/srep28951
- Sachs N, De Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. janv 2018;172(1‑2):373-386.e10.
- Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J, et al. Patient‐Derived Organoids Can Guide Personalized‐Therapies for Patients with Advanced Breast Cancer. Adv Sci [Internet]. nov 2021 [cité 16 juill 2025];8(22). Disponible sur: https://onlinelibrary.wiley.com/doi/10.1002/advs.202101176
- Saeki S, Kumegawa K, Takahashi Y, Yang L, Osako T, Yasen M, et al. Transcriptomic intratumor heterogeneity of breast cancer patient-derived organoids may reflect the unique biological features of the tumor of origin. Breast Cancer Res [Internet]. 21 févr 2023 [cité 16 juill 2025];25(1). Disponible sur: https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-023-01617-4
- Buchholz MB, Scheerman DI, Levato R, Wehrens EJ, Rios AC. Human breast tissue engineering in health and disease. EMBO Mol Med. 23 août 2024;16(10):2299‑321.
- Xu R, Yin P, Wei J, Ding Q. The role of matrix stiffness in breast cancer progression: a review. Front Oncol [Internet]. 17 oct 2023 [cité 16 juill 2025];13. Disponible sur: https://www.frontiersin.org/articles/10.3389/fonc.2023.1284926/full
- Jin Q, Liu G, Li S, Yuan H, Yun Z, Zhang W, et al. Decellularized breast matrix as bioactive microenvironment for in vitro three‐dimensional cancer culture. J Cell Physiol. avr 2019;234(4):3425‑35.
- Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell. déc 2018;175(7):1972-1988.e16.
- Firatligil-Yildirir B, Yalcin-Ozuysal O, Nonappa. Recent advances in lab-on-a-chip systems for breast cancer metastasis research. Nanoscale Adv. 2023;5(9):2375‑93.
- Yang J, Qu J, Zhang M, Li X, Jiang Q, Kang J, et al. Dynamic culture system advances the applications of breast cancer organoids for precision medicine. Sci Rep. 14 mars 2025;15(1):8852.
- Testa M, Gaggianesi M, D’Accardo C, Porcelli G, Turdo A, Di Marco C, et al. A Novel Tumor on Chip Mimicking the Breast Cancer Microenvironment for Dynamic Drug Screening. Int J Mol Sci. 25 janv 2025;26(3):1028.
FAQ
Two-dimensional cultures of immortalised breast-cancer cell lines have been used for screening and pathway discovery. These models have systemic limitations. Standard 2D plasticware is used. This material enforces a flattened morphology on the cells. This setup disrupts apico-basal polarity and disrupts cell-to-matrix interactions. Gradients for oxygen, nutrients, and drug-diffusion are erased. As a consequence, the transcriptomic and metabolic profiles of the cells deviate from those found in primary tumours. Context-dependent drug responses are dampened by these changes. Furthermore, continuous passaging fosters genetic drift. This means independent cell stocks can diverge markedly in growth rate and drug susceptibility, which undermines reproducibility.
Cell-line–derived xenografts (CDX) are an in vivo extension of 2D cultures. When transplanted, CDX tumours do copy the parental-line genetics. They propagate, however, as uniform epithelial masses. These masses are devoid of the stromal, vascular, and immune elements that shape drug penetration in vivo. Most of the index cell lines are also derived from late-stage pleural effusions. This origin makes CDXs ill-suited for probing early oncogenic events. They are also not good for studying the emergence of endocrine resistance. The continuous in vitro passaging before engraftment also causes genetic drift and clonal selection. This process produces divergent subclones. It narrows the biological heterogeneity that was originally present in the patient tumours.
The architectural change from a flat monolayer to a three-dimensional culture re-programmes how cells respond to therapy. Studies showed that lines grown as dense multicellular spheroids were markedly less susceptible to paclitaxel or doxorubicin than their 2D counterparts. This observation mirrors the dormancy and hypoxia encountered in vivo. One study of thirteen triple-negative breast-cancer lines reported two- to ten-fold increases in IC₅₀ values for epirubicin, cisplatin, and docetaxel in 3D spheroids versus 2D culture. It was also found that trastuzumab efficacy against HER2-positive cells declines sharply under 3D conditions. These discordant drug responses arise because 3D models reinstate gradient-driven hypoxia and integrin-mediated mechanotransduction.
The different drug responses in 3D models are explained by two main factors. These factors are described as extrinsic and intrinsic. Extrinsically, extracellular-matrix surrogates like hydrogels are used. These materials reinstate microenvironmental variables. These include gradient-driven hypoxia and paracrine crosstalk. These variables are known to modulate proliferation and apoptotic thresholds. Intrinsically, patient-derived breast cancer organoids (PDOs) are used. PDOs conserve the genomic, transcriptomic, and histological complexity of their primary tumours. This conservation is much more faithful than in established cell lines. These models are seen as a more stringent and clinically resonant platform for pre-clinical drug discovery.
Patient-derived organoids (PDOs) are reported to conserve the complexity of their primary tumours with high fidelity. One study involving over one hundred breast-cancer organoid lines was led by Sachs et al.. It was shown that the organoids retained their copy-number landscapes and receptor phenotypes over twenty passages. Another study demonstrated that the pharmaco-phenotypes of PDOs taken from drug-resistant patients were able to predict subsequent clinical responses. Furthermore, intratumoral heterogeneity is preserved ex vivo. A single-cell analysis by Saeki et al. confirmed this. Three to six transcriptionally distinct cancer-cell states were uncovered within individual organoid lines. This included rare epithelial-to-mesenchymal intermediates.
The extracellular matrix (ECM) is a component of the tumour microenvironment. In the healthy gland, it includes a compliant, laminin-rich basement membrane embedded within adipose-fibroblast stroma. This architecture is needed for normal function, such as lactational capacity. Tumorigenesis rewires this matrix in its composition and mechanics. Changes include the loss of polarity-sustaining laminin isoforms and the up-regulation of matrix metalloproteinases (MMPs). Pathological deposition of fibrillar collagens also occurs, which cross-links and stiffens the stroma. Normal breast tissue has an elastic modulus of about 0.2 kPa, but invasive lesions can exceed 4 kPa. This biophysical shift is known to accelerate epithelial–mesenchymal transition (EMT).
Yes, platforms can now embed cancer cells within biomimetic matrices. These matrices mirror disease-specific cues. In one study, matched normal and tumour biopsies were decellularised. This process generated human breast ECM scaffolds. MCF-7 cells were then reseeded onto these scaffolds. The tumour-derived scaffolds were observed to induce epithelial–mesenchymal transition (EMT) and proliferative expansion. In contrast, the normal scaffolds promoted apoptosis. This provided direct evidence that the biochemical and mechanical properties of the ECM dictate cell fate. Integrating native-like matrices into organoid culture is therefore considered important.
Yes, patient-derived breast cancer organoids (PDOs) can retain endogenous immune and stromal elements. This retention is possible when they are cultured in air–liquid or microfluidic formats. A study by Neal et al. demonstrated this capability. It was shown that PDOs established with an air–liquid interface preserve tumour-infiltrating lymphocytes (TILs). The T-cell receptor repertoire of these TILs was also maintained. This setup allows for the in vitro interrogation of PD-1/PD-L1 blockade. The testing can be done within an intact tumour-immune ecosystem. Coupling such immunocompetent PDOs with ECM-engineered scaffolds is a new way to study how different factors interact.
Microfluidic organ-on-chip (OoC) technology extends the advantages of static organoids. Continual perfusion is added. Controllable shear stress and compartmentalized co-culture are also added. These systems can be used for different purposes. Breast-cancer metastasis chips, for example, recreate endothelial barriers and chemokine gradients. This allows for tracking real-time invasion and the arrest of circulating-tumour-cells. Tumour-infiltrating lymphocyte analysis can also be permitted under defined oxygen tension. Dynamic flow has been shown to accelerate organoid growth. It also preserves drug-response fidelity. Culture timelines can be trimmed from weeks to days.
Yes, 2D cultures remain useful for specific applications. A large panel of immortalised breast-cancer cell lines exists. These lines are classified into subsets that mirror clinical subtypes, such as luminal A, luminal B, and HER2-enriched. Because well-characterized oncogenic drivers like PIK3CA, TP53, and ESR1 are retained, these cultures are still used for deciphering oncogenic signalling. They are also used for studying synthetic-lethal dependencies and endocrine-resistance mechanisms. Their ease of propagation and genetic tractability are convenient properties. Their compatibility with robotic platforms has made them a cornerstone of compound library screening and CRISPR screens. Many lines can also form cell-line–derived xenografts (CDX), which offer an economical in vivo extension for some studies.