CherryTemp —
Fast temperature shift during live cells imaging device
- Applicable for C. elegans, Phase separation, S. pombe, S.cerevisiae and more.
- Compatible inverted and upright microscope
- Usable with oil & water immersion and dry air objectives
- Ultrafast & precise temperature shift [10′ sec - 5 to 45°C]
- 0,2°C precision and below ambient temperature control
CherryTemp - Applications

Ebook download
—
complete guide to select the right Temperature control system for microscopy
Overview
CherryTemp is a temperature controller based on two independent Peltier channels, allowing for very accurate temperature control and ultra-fast shifts from 5°C to 45°C.
A thermalization liquid circulates from a reservoir to the heat exchangers (heating/cooling Peltier elements) where it is thermalized to a target temperature. Once thermalized, this liquid can be quickly injected to a microfluidic device (CherryTemp chip) mounted near the sample. Because of the small thermal mass (the distance between the microfluidic chip and the sample), the temperature transfer is very fast (less than 10 seconds) and of unprecedented accuracy due to a feedback loop control of the room temperature and the heat-sink played by immersion objective lenses.
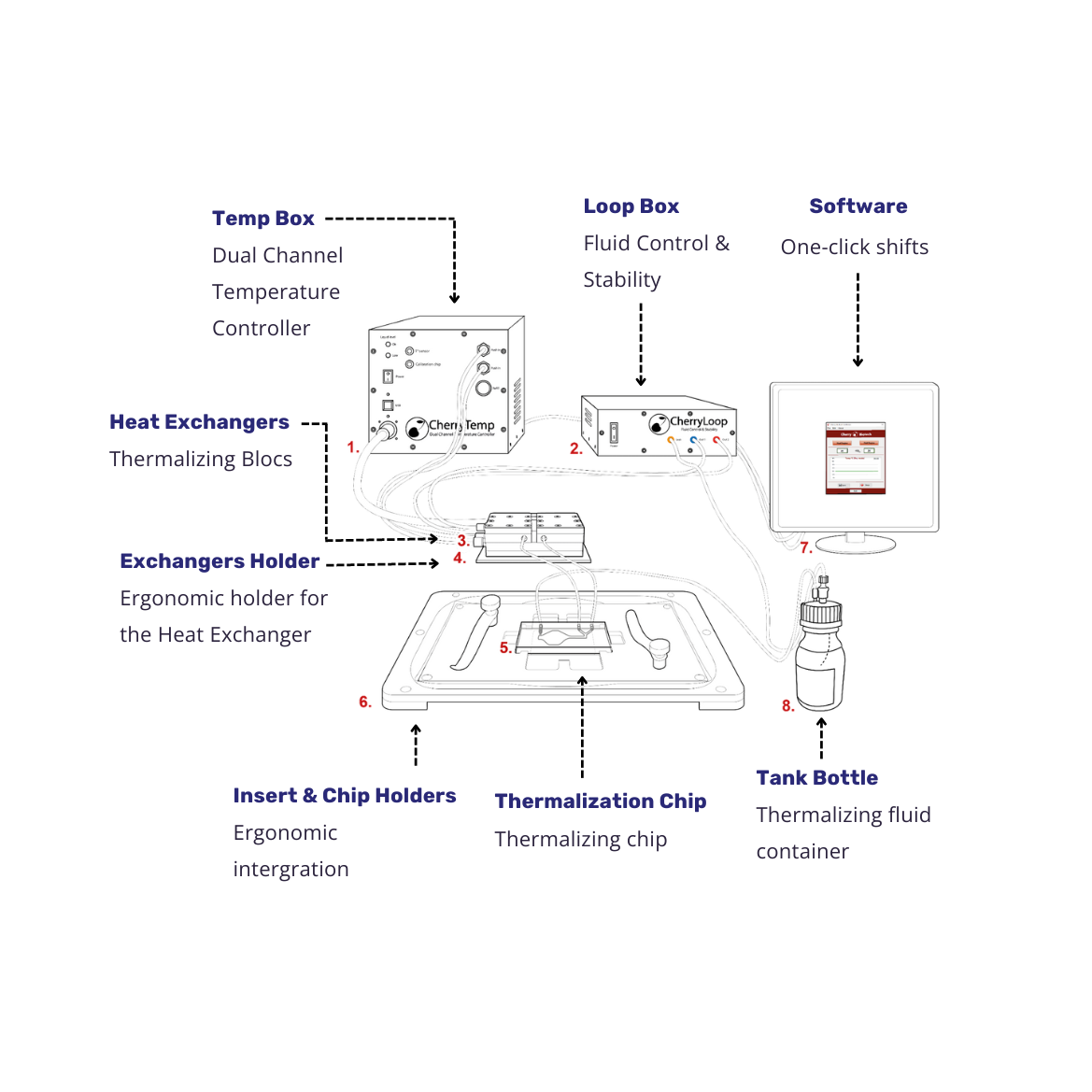
Specifications

User Interface
Cherry Temp’s user interface offers several advantages, including:
- One-click setting
- Optimized window for easy integration with other software
- Simple navigation
These features make it simple and efficient for users to set up and operate the system, streamlining the experimental process and enhancing productivity.
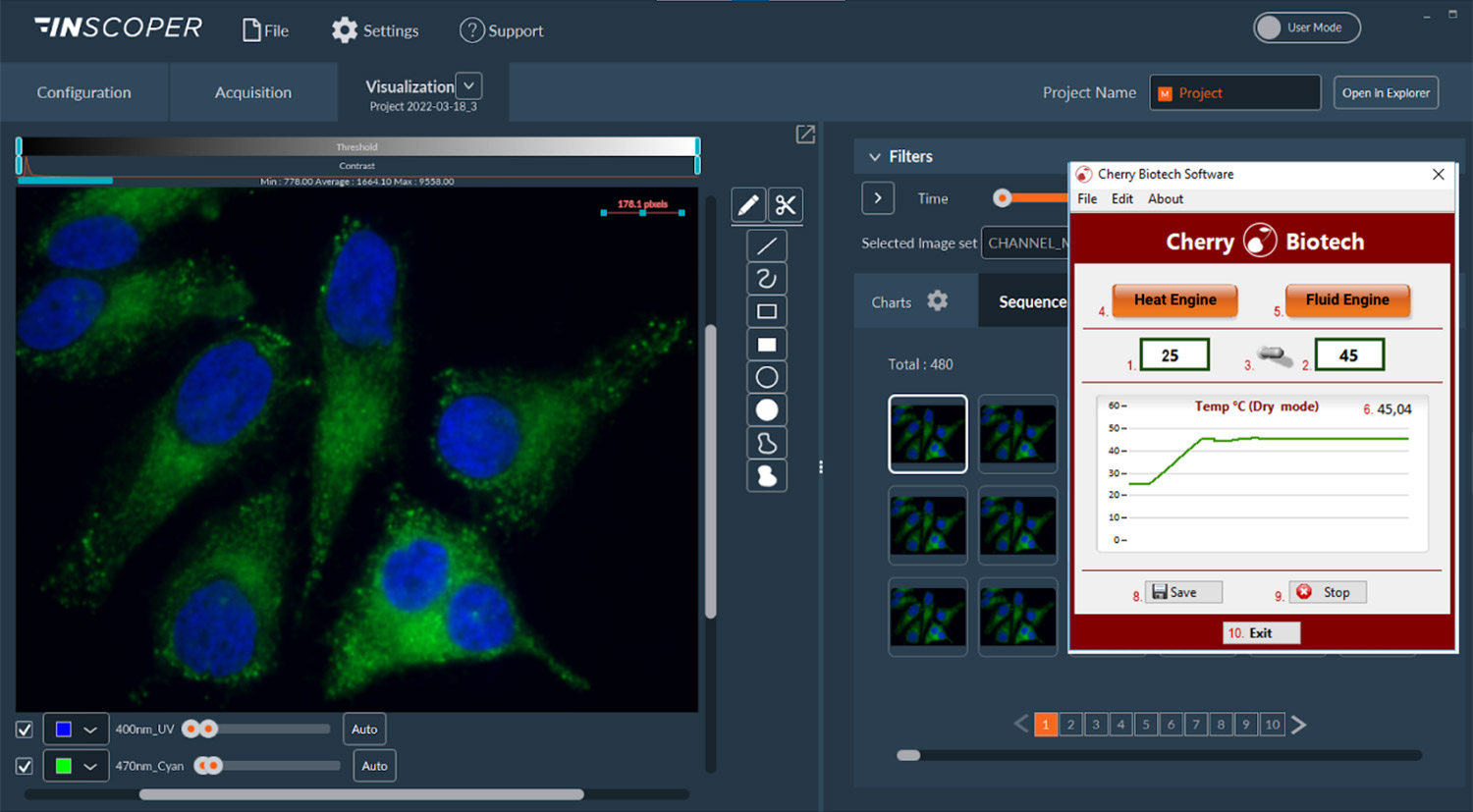
Image: CherryTemp’s interface compatible with INSCOPER software (note: image use authorized by owners)
FAQ
It is a strong point of our CherryTemp system to ensure temperature precision but also homogeneity and stability over time. The temperature homogenous on the sample area with a 0.2°C precision given that the distance between our thermalization unit and your sample remains under 0.5mm (500µm).
This is excellent specifications compared to most systems where there can be several degrees difference and big homogeneity problems, which can become worse with the heat sink of immersion objectives or the extra heating of halogen lamps.
We use a combination of several methods for extra accuracy, combining a thermal camera to validate all manufactured systems, micro-electrodes deposition at the sample location (see 4-points electrode deposit method), numerical algorithms and biological confirmation experiments as a last check (thermosensitive mutants for instance).
Our innovation relies on coupling a temperature calibration of our systems and a smart algorithm taking into account all the sources of heat lost: the room temperature fluctuations and the heat sink played by the objective lens.
We performed several experiments and set up two specific calibrations whether an immersion objective was in contact with the coverslip or not. Our software integrates these data and calculates a correcting factor depending on the type of objective lense you will use for your experimentation. As a consequence, the temperature you set in the software is exactly the one that is experienced by your cells.
No, we constantly monitor the temperature of the objective lense with a sensor. A feedback loop adjusts in real-time our algorithm to ensure a 0.3°C stability whatever are the temperature fluctuations.
Thanks to our dual-channel heat exchangers, a shift between two temperatures takes few seconds (10 seconds) and does not rely on the starting or ending setting. It means that it takes 10 seconds both for a 5°C delta change or a 40°C delta change.
This is a physical parameter that no system can avoid but which is absent when using immersion objective lenses. This phenomenon is limited when a robust air condition is used in the microscope room.
We worked to limit evaporation by providing a dedicated spacer (a silicon elastomer) that you simply put on the coverslip. It creates a well where cells and medium can be pipetted. When our thermalization chip comes on top, it creates a closed chamber which limits evaporation over time.
Recent publications feature CherryTemp
Some significative C. elegans articles :
- Dumont et al., 2023, Kinetochore component function in C. elegans oocytes revealed by 4D tracking of holocentric chromosomes, Nature Communications
- Glauser et al., 2023, Multisite regulation integrates multimodal context in sensory circuits to control persistent behavioral states in C. elegans, Nature Communications
- Bowerman et al., 2021, A genetic screen for temperature-sensitive morphogenesis-defective Caenorhabditis elegans mutants, G3
- Seydoux et al., 2019, A gel phase promotes condensation of liquid P granules in Caenorhabditis elegans embryos, Nature Structural & Molecular Biology
- Overholtzer et al., 2019, Entosis Controls a Developmental Cell Clearance in C. elegans, Cell Reports
More C. elegans article published with our CherryTemp system: Read more
Some significative phase separation article:
- Elbaum-Garfinkle et al., 2022, Liquid to solid transition of elastin condensates, PNAS
- Elbaum-Garfinkle et al., 2020, Tunable multiphase dynamics of arginine and lysine liquid condensates, Nature Communications
- Seydoux et al., 2019, A gel phase promotes condensation of liquid P granules in Caenorhabditis elegans embryos, Nature Structural & Molecular Biology
- Kimble et al., 2019, Dynamics of Notch-Dependent Transcriptional Bursting in Its Native Context, Developmental Cell
- Rosen et al., 2018, Nuclear Import Receptor Inhibits Phase Separation of FUS through Binding to Multiple Sites, Cell
More Phase separation articles published with our CherryTemp system: Read more
What Users Say About CherryTemp

“The Cherry system is now in full use in my lab and it is working beautifully on its own. The Cherry system is great for stably maintaining temperature on our microscopes”
Dr. Julie Canman Columbia University Medical Center, USA
“CherryTemp is a one-of-a kind device for the study of thermosensitive mutants and cytoskeleton dynamics. Its flexibility makes it very easy to adapt to various geometries, from cells to tissue explants or even whole animals !”
Dr. Emmanuel Derivery MRC Lab of Molecular Biology, UK
“CherryTemp rapidly became one of our favorite piece of equipment in the lab. It’s easy to use and works fast and well. The Cherry Biotech team is always super helpful.”
Dr. Julien Dumont INSTITUT JACQUES MONOD, FRANCE
“The Cherry Temp is perfect for use in imaging core facilities. Its design allows its installation on a wide panel of upright and inverted microscopes and easy training of users thus, promoting development of innovating research project. The customer service is always helpful and responsive.”
Dr. Stéphanie Dutertre BIOSIT LABORATORY, France
“CherryTemp allows us to perform temperature shift experiments with unprecedented speed and precision with C. elegans embryos. It is very well designed and easy to use. Moreover, we found the the customer service at Cherry Biotech to be very responsive and competent.”
Prof. Pierre Gönczy EPFL, SWITZERLAND
“CherryTemp was the perfect solution for us to combine precisely controlled temperature stimuli and live cell imaging in our functional studies on thermosensory neurons in C. elegans adult animals. The system is straightforward to use and we were impressed by the responsiveness of the Cherry Biotech customer service team and by the quality of the support provided.”
Prof. Dominique Glauser UNIVERSITY OF FRIBOURG, SWITZERLANDThey Trust Us




















" The Cherry Temp system was essential to acquiring some of our key data on the temperature-dependence of FUS phase separation. The system worked extremely well in these biochemical analyses. It was easy to use and robust, and required only small amounts of material. ”
Prof. Michael Rosen UT SOUTHWESTERN, USA