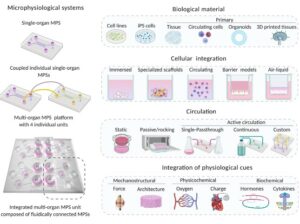
Adipose tissue organoid technology is reshaping metabolic research. From scaffold-free spheroids to hydrogel-based 3D adipose tissue model, these 3D in vitro models capture endocrine and immune cues lost in 2D. Recognized as microphysiological systems within emerging New Approach Methodologies (NAMs), adipose tissue organoids platforms promise safer, faster drug screens.
Typical Protocol for Adipose Tissue Organoids Development
Adipose organoid construction begins with cell sourcing. Platforms now employ pluripotent stem cells (hiPSC/hESC), adipose-derived stem cells (ASCs), whole stromal-vascular fractions (SVF) from white or brown depots, and immortalised pre-adipocyte lines such as 3T3-L1 or C3H10T1/2. Each source carries a distinct bias: SVF-BAT is already brown-skewed, whereas PSC- and ASC-derived progenitors remain flexible until guided by differentiation cues.
Whatever the origin, adipogenesis funnels through the transcriptional pair C/EBPα–PPARγ(1). A basal white-fat cocktail (insulin + dexamethasone + IBMX + rosiglitazone) activates this axis, producing unilocular, leptin-rich adipocytes within two weeks. Brown or beige programmes add thermogenic drivers: triiodothyronine, cAMP analogues, BMP7 or β-adrenergic agonists prolong PPARγ activity, stabilise PRDM16/EBF2 and elevate UCP1, generating multilocular heat-producing cells(1). Thus, white differentiation rests on the C/EBP→PPARγ pathway, whereas brown induction layers a thermogenic module over the same core.
Three-dimensional assembly follows one of two routes. Scaffold-free methods (hanging drops, microwell arrays or ultra-low-attachment (ULA) plates) let progenitors self-aggregate; ULA “is the most widely used scaffold-free technique due to its automation, simplicity and high throughput”(1). Resulting spheroids (≈100–400 µm) are ideal for screening but may develop hypoxic centres during extended culture. Hydrogel-based protocols embed cells or spheroids in GelMA, PEG-RGD, fibrin or decellularised adipose ECM. Such 3D porous or permeable biomaterials promote the recapitulation of the structure and functionality of native tissues(2), while tunable stiffness and porosity steer lineage bias, stiffer, porous gels enhance brown/beige thermogenesis; softer fibrin favours large white cells. Hybrid workflows increasingly combine both approaches, forming spheroids first, then transferring them into oxygen-permeable hydrogels for long-term perfusion and vascular sprouting.
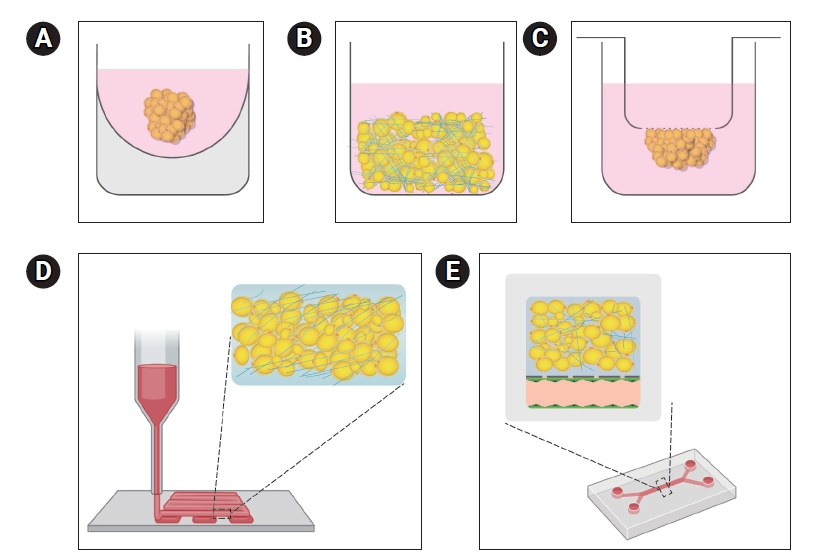
Figure 1
Caption: In vitro adipocyte culture platforms. (A) Adipocyte spheroid culture, (B) three-dimensional (3D) culture with biomaterials, (C) membrane mature adipocyte aggregate culture, (D) 3D bioprinting, (E) microfluidic physiological system. From Heejeong Yoon, Tae-Eun Park, Engineered adipose tissue platforms: recent breakthroughs and future perspectives, Organoid, January 25, 2023, DOI: https://doi.org/10.51335/organoid.2023.3.e1
Physiological Relevance of Adipose Tissue Organoid Models in Metabolic Research
Adipose tissue organoids offer an advanced human-relevant platform for metabolic research by faithfully replicating the multicellular complexity and dynamic physiology of native fat depots. Unlike 2D monolayers that generate small, multilocular adipocytes with limited lipid storage and poor endocrine function, organoids (particularly those assembled using scaffold-free or hydrogel-based techniques) mature into large, unilocular, lipid-laden adipocytes embedded within a self-organising stromal–vascular–immune niche. This spatial architecture more closely mirrors in vivo white and brown adipose tissue, allowing for more accurate disease modeling.
A critical innovation in 3D culture systems is the inclusion of endothelial elements, either through spontaneous sprouting from stromal-vascular fractions or through the integration of microvascular fragments. These vascular-like structures create a physiologically relevant growth environment that accelerates adipocyte maturation and promotes lipid droplet expansion. In particular, the formation of HUVAS (human unilocular vascularized adipocyte spheroids) results in markedly enhanced leptin and adiponectin secretion relative to 2D monolayers, indicating superior endocrine fidelity. These adipokines, key regulators of energy homeostasis, respond to lipotoxic conditions by shifting the adiponectin-to-leptin ratio, faithfully reproducing the endocrine imbalance observed in obesity and insulin resistance.
Immune components further enhance the physiological relevance of these models. The retention of resident macrophages and mast cells from SVF enables immune–metabolic crosstalk, an essential feature of adipose dysfunction in obesity. Lipidomic studies confirm that immune-competent organoids reproduce diet-induced changes in neutral lipid and phospholipid pools more accurately than traditional models, reflecting their deeper metabolic integration.
Functionally, both white and thermogenic variants display hallmark behaviours. Brown and beige adipose organoids express UCP1, engage in uncoupled mitochondrial respiration, and respond to β-adrenergic stimulation with glucose uptake and lipolysis, confirming that their thermogenic machinery is intact. In contrast, white adipose organoids exhibit the expected unilocular morphology, store triglycerides efficiently, and respond to insulin, until exposed to chronic fatty-acid overload, which induces insulin resistance and pro-inflammatory cytokine release. These dynamic phenotypes position organoids as reliable microphysiological systems, aligning them with the FDA’s expanding list of qualified New Approach Methodologies.
Additionally, the 3D environment allows for ECM deposition and remodeling. Cells within organoids synthesize native matrix proteins or dynamically reshape hydrogel scaffolds with tunable stiffness and porosity. This feature makes them ideal for studying how mechanical and biochemical cues guide depot-specific adipogenesis and metabolic flexibility. The compatibility of these systems with real-time metabolic assays enables quantitative analysis of mitochondrial function, glucose flux, and immune signaling networks. These data-rich outputs are difficult to obtain from animal models and virtually inaccessible in 2D cultures.
Altogether, adipose tissue organoids represent a physiologically robust and highly versatile model for dissecting the pathophysiology of metabolic disease, advancing therapeutic screening, and supporting regulatory decision-making with human-specific data(1–3).
Research and Therapeutic Use Cases for Adipose Tissue Organoids Platforms
As increasingly sophisticated 3D in vitro adipose tissue constructs, adipose tissue organoids have moved from proof-of-concept curiosities to versatile microphysiological systems that underpin a wide spectrum of translational work. In pre-clinical pharmacology, these 3D in vitro models are already being deployed in 96- and 384-well formats for medium-throughput phenotypic screens that probe adipogenesis, insulin sensitivity, lipolysis and thermogenic activation under human-relevant conditions. Their unilocular architecture, intact stromal–vascular niche and endocrine competence generate drug-response signatures that outperform 2D monolayers and may flag off-target toxicity missed in rodent assays, an outcome squarely aligned with the FDA’s push toward New Approach Methodologies.
Disease-modelling applications are equally compelling. By exposing white adipose organoids to chronic free-fatty-acid overload or glucocorticoid excess, researchers can induce insulin resistance, adipokine dysregulation and low-grade inflammation that mirror the pathophysiology of obesity and metabolic syndrome. Brown or beige organoids, conversely, permit dissection of mitochondrial uncoupling defects and UCP1 regulation in rare lipodystrophies or age-related thermogenic decline. Because the constructs preserve immune and endothelial compartments, they allow quantification of cytokine crosstalk, ECM remodeling and angiogenic responses; parameters that are critical to understanding how adipose tissue contributes to systemic cardiometabolic risk.
Personalized medicine avenues build on the ease with which patient-specific stromal-vascular fractions or hiPSC lines can be channeled into organoid protocols. Such bespoke 3D adipose tissue models capture an individual’s genetic and epigenetic landscape, enabling ex-vivo testing of anti-obesity or anti-diabetic compounds before clinical exposure, an approach that accelerates target de-risking while remaining entirely human-centric.
Regenerative-therapy research extends the technology. Transplantation of vascularized brown adipose organoids into obese or diabetic rodent models improves glucose tolerance and energy expenditure(4). Although clinical translation will require scalable GMP biomanufacturing and rigorous safety testing, these proof-of-concept studies highlight the potential of organoids as living implants that augment a patient’s own metabolic capacity.
Finally, the platform is gaining traction in toxicology and environmental-health sciences. Because adipose tissue functions as both an endocrine organ and a reservoir for lipophilic pollutants, organoids furnish a controllable setting to study how endocrine disruptors, persistent organic pollutants or novel food additives accumulate and interfere with adipocyte biology. Integrated lipidomics and high-content imaging allow simultaneous measurement of xenobiotic burden, adipokine secretion and mitochondrial health, delivering a systems-level view that traditional assays cannot match.
Challenges and Future Directions for Adipose Tissue Organoids and Microphysiological Systems
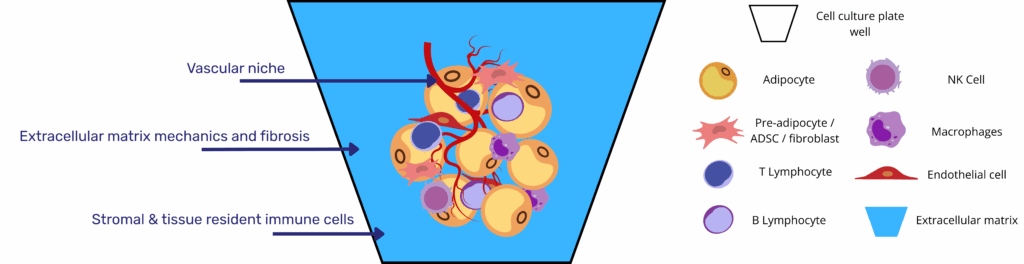
Over the last decade, 3D culture protocols have finally delivered human‐relevant adipose tissue organoids that contain large, unilocular adipocytes. Yet, as the field moves from “adipocytes in a dish” toward true adipose tissue organoid microphysiological systems, three layers of microenvironmental control still limit physiological and pathophysiological fidelity.
- Vascularization and oxygen supply
Capillary proximity is a prerequisite for lipid-droplet unilocularity, endocrine function, and the study of angiogenesis–adipocyte crosstalk that drives obesity-associated inflammation. Human unilocular vascularized adipocyte spheroids (HUVAS) elegantly show that endothelial niches accelerate maturation and, when loaded with exogenous lipid, recapitulate hypertrophy-linked insulin resistance(5). Despite such progress, static spheroids rely on diffusion and rapidly develop hypoxic cores. Engineered perfusable microvessels capable of delivering physiological shear stress and of recruiting circulating immune cells are therefore a primary engineering target. - Extracellular-matrix mechanics and fibrosis
White adipose tissue stiffens two- to three-fold in obesity, and elevated matrix stiffness amplifies profibrotic and pro-inflammatory signaling in stromal progenitors. Re-creating this dynamic range in vitro requires biomaterials with tunable viscoelasticity and integrin engagement; otherwise, adipocytes stay multilocular and insulin-sensitive. Recent reviews highlight how harnessing synthetic or ECM-derived scaffolds with adjustable cross-linking can model the transition from healthy to fibrotic fat, providing a quantitative handle on mechano-inflammation coupling(3). - Stromal and immune complexity
Adipose tissue is more than adipocytes: mesenchymal stem cells, fibroblasts, tissue-resident macrophages, and lymphocytes collectively sculpt adipokine output and metabolic plasticity. Conventional organoid media, however, often strip away immune and stromal cues. Incorporating defined stromal fractions has already improved adipogenesis and lipid handling in spheroids(6), but future platforms must allow controlled recruitment and perfusion of immune subsets to interrogate obesity-induced low-grade inflammation in real time.
Microfluidic culture adds the missing dimensions of flow, shear, and inter-organ exchange. Adipose microtissue-on-chip platforms now automate spheroid formation, differentiation, and longitudinal proteomics under precisely programmed media oscillations, proof that large-scale integration chips can sustain adipocytes for weeks without serum(7). Continuous perfusion establishes:
- Shear‐conditioned endothelial barriers that align and form tight junctions, enabling leukocyte trafficking and angiogenesis assays.
- Gas control, essential to model regional adipose hypoxia that initiates HIF-driven inflammation.
- Dynamic nutrient waves (glucose, fatty acids) that mimic feast-fast cycles and unmask metabolic inflexibility.
Going further, coupling adipose chambers to liver, muscle, or pancreatic islet modules capture systemic crosstalk. An adipose–liver human-on-a-chip has already reproduced non-alcoholic fatty-liver disease (NAFLD) hallmarks; steatosis, cytokine spill‐over, and drug responses unobtainable in monocultures(8). Such multi-organ circuitry will be indispensable for tackling complex co-morbidities like type 2 diabetes.
The next generation of adipose tissue organoid microphysiological systems must integrate:
- Perfused microvasculature to sustain long‐term adipocyte viability and study angiogenic remodeling.
- Adaptive ECM scaffolds that tune stiffness and biochemical composition across healthy, fibrotic, and inflammatory states.
- Modular stromal and immune compartments that can be added or removed to dissect cell-type–specific contributions.
- Multi-organ interconnectivity for whole-body metabolism and drug-safety assessment.
Resources
- Liu X, Yang J, Yan Y, Li Q, Huang RL. Unleashing the potential of adipose organoids: A revolutionary approach to combat obesity-related metabolic diseases. Theranostics. 2024;14(5):2075‑98.
- Yoon H, Park TE. Engineered adipose tissue platforms: recent breakthroughs and future perspectives. Organoid. 25 janv 2023;3:e1.
- Contessi Negrini N, Pellegrinelli V, Salem V, Celiz A, Vidal-Puig A. Breaking barriers in obesity research: 3D models of dysfunctional adipose tissue. Trends Biotechnol. mai 2025;43(5):1079‑93.
- Nguyen HP, An K, Ito Y, Kharbikar BN, Sheng R, Paredes B, et al. Implantation of engineered adipocytes suppresses tumor progression in cancer models. Nat Biotechnol [Internet]. 4 févr 2025 [cité 4 avr 2025]; Disponible sur: https://www.nature.com/articles/s41587-024-02551-2
- Ioannidou A, Alatar S, Schipper R, Baganha F, Åhlander M, Hornell A, et al. Hypertrophied human adipocyte spheroids as in vitro model of weight gain and adipose tissue dysfunction. J Physiol. févr 2022;600(4):869‑83.
- Baganha F, Schipper R, Hagberg CE. Towards better models for studying human adipocytes in vitro. Adipocyte. 31 déc 2022;11(1):413‑9.
- Compera N, Atwell S, Wirth J, Von Törne C, Hauck SM, Meier M. Adipose microtissue-on-chip: a 3D cell culture platform for differentiation, stimulation, and proteomic analysis of human adipocytes. Lab Chip. 2022;22(17):3172‑86.
- Slaughter VL, Rumsey JW, Boone R, Malik D, Cai Y, Sriram NN, et al. Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical therapeutic efficacy evaluation. Sci Rep. 23 juin 2021;11(1):13159.
FAQ
The construction of adipose organoids starts with the sourcing of cells. Different platforms use various cell types. Pluripotent stem cells, including hiPSC and hESC, may be used. Adipose-derived stem cells (ASCs) are also a common source. From tissue, whole stromal-vascular fractions (SVF) can be taken from white or brown fat depots. Immortalised pre-adipocyte lines, such as 3T3-L1, are another option. A distinct bias is associated with each cell source. For example, SVF from brown adipose tissue is already skewed toward a brown fat profile. In contrast, progenitors derived from PSCs and ASCs stay flexible. They are guided by specific differentiation cues applied in the culture.
Adipogenesis is directed by the transcriptional pair C/EBPα–PPARγ, regardless of the cell origin. A specific cocktail is used for developing basal white fat. This mixture contains insulin, dexamethasone, IBMX, and rosiglitazone. This combination activates the C/EBPα–PPARγ axis. The result is the production of unilocular, leptin-rich adipocytes within a two-week period. For brown or beige fat programmes, different thermogenic drivers are added to the process. These drivers may include triiodothyronine, BMP7, or β-adrenergic agonists. The activity of PPARγ is prolonged by these substances. PRDM16 and EBF2 are stabilised. UCP1 levels are also elevated. This process generates multilocular, heat-producing cells.
Two main routes are followed for three-dimensional assembly. The first route uses scaffold-free methods. These methods include hanging drops or microwell arrays. Ultra-low-attachment, or ULA, plates are also used. This ULA method is reported as widely used because of its simplicity and suitability for automation. These techniques permit progenitors to self-aggregate. Spheroids of 100 to 400 micrometres are formed. Hypoxic centres may be developed by these spheroids during long culture periods. The second route involves hydrogel-based protocols. In these methods, cells or spheroids are embedded within a substance. Materials like GelMA, fibrin, or decellularised adipose ECM are used. These biomaterials are porous and permeable. They support the formation of structures that resemble native tissues.
Adipose tissue organoids are considered a human-relevant platform. They are seen to replicate the multicellular complexity and physiology of native fat depots. Conventional 2D monolayers are different. They typically generate small, multilocular adipocytes. These cells have restricted lipid storage capacity and poor endocrine function. In contrast, 3D organoids mature into large, unilocular adipocytes that are laden with lipids. These cells are embedded within a self-organising niche. This niche contains stromal, vascular, and immune components. This spatial architecture is a closer representation of in vivo white and brown adipose tissue. More accurate disease modelling is permitted by this arrangement.
The inclusion of endothelial elements is an innovation in 3D culture systems. A growth environment that is physiologically representative is created by these vascular-like structures. Adipocyte maturation is accelerated. Lipid droplet expansion is also promoted. A specific formation, human unilocular vascularized adipocyte spheroids (HUVAS), shows these benefits. A large increase in leptin and adiponectin secretion is observed in HUVAS when compared to 2D monolayers. This indicates a higher degree of endocrine fidelity. These adipokines are regulators of energy homeostasis. They are observed to respond to lipotoxic conditions. The adiponectin-to-leptin ratio is shifted. This shift reproduces the endocrine imbalance seen in obesity and insulin resistance.
Thermogenic variants of adipose organoids display specific behaviours in research. Brown and beige adipose organoids are shown to express UCP1. They also engage in uncoupled mitochondrial respiration. A response to β-adrenergic stimulation is also observed. This response includes glucose uptake and lipolysis. These findings confirm that their thermogenic machinery is intact. This is different from white adipose organoids. White adipose organoids show a unilocular morphology and store triglycerides. They respond to insulin. This response changes when they are exposed to chronic fatty-acid overload. This exposure induces insulin resistance. Pro-inflammatory cytokine release is also triggered. These distinct phenotypes allow for different metabolic processes to be studied.
Adipose organoids are applied in pre-clinical pharmacology as 3D in vitro models. They are being deployed in 96- and 384-well formats. These formats are used for medium-throughput phenotypic screens. These screens are designed to probe several processes. Adipogenesis, insulin sensitivity, lipolysis, and thermogenic activation are examined under human-relevant conditions. The models have a unilocular architecture. They also possess an intact stromal–vascular niche and endocrine competence. These features permit the generation of drug-response signatures. These signatures are reported to be superior to those from 2D monolayers. Off-target toxicity that might be missed in rodent assays may also be flagged by these models.
Adipose organoids are used for disease-modelling applications. White adipose organoids can be exposed to specific conditions. Chronic free-fatty-acid overload or glucocorticoid excess are examples of these conditions. These exposures are used to induce states such as insulin resistance. Adipokine dysregulation and low-grade inflammation can also be induced. These states are reported to mirror the pathophysiology of obesity and metabolic syndrome. Brown or beige organoids are used for different purposes. They permit the dissection of mitochondrial uncoupling defects. The regulation of UCP1 in rare lipodystrophies or in age-related thermogenic decline can also be studied.
Static organoids face limitations related to vascularization and oxygen supply. Capillary proximity is a requirement for unilocular lipid-droplet formation and proper endocrine function. Static spheroids, however, must rely on diffusion for nutrients and oxygen. This reliance on diffusion means that hypoxic cores are developed rapidly. This lack of a proper vascular network is a problem for long-term culture and function. Human unilocular vascularized adipocyte spheroids, or HUVAS, show that endothelial niches accelerate maturation. The development of engineered, perfusable microvessels is noted as a primary engineering target. These vessels would be able to deliver physiological shear stress. They might also recruit circulating immune cells, further improving the model’s fidelity.
The mechanics of the extracellular matrix, or ECM, present a limitation. In obesity, white adipose tissue is known to stiffen. This elevated matrix stiffness increases profibrotic and pro-inflammatory signaling in stromal progenitors. The recreation of this dynamic stiffness range in vitro is a challenge. It requires biomaterials that have tunable viscoelasticity. Proper integrin engagement is also needed. If these conditions are not met, the adipocytes may stay multilocular and overly insulin-sensitive. This state does not represent the diseased condition. Synthetic or ECM-derived scaffolds with adjustable cross-linking are being examined. These materials can be used to model the transition from healthy to fibrotic fat.