Breast cancer remains the most frequently diagnosed malignancy in women worldwide and a leading cause of cancer-related mortality. Yet the attrition rate of oncology drugs in late-stage trials is high, exposing the limitations of conventional pre-clinical tools and raising the question of the use of breast cancer organoids as alternative to animal models. Two-dimensional cell lines lack tumour architecture, and even advanced mouse models cannot fully reproduce human mammary physiology, immune crosstalk or pharmacokinetics. This translation gap fuels an ethical and scientific push toward animal testing alternatives able to recapitulate complex disease biology while reducing animal use. Among emerging options, the breast cancer organoid has gained prominence: primary tumour cells embedded in extracellular matrix self-organise into 3D breast cancer models that preserve histology and genomic heterogeneity. When integrated with stromal or immune components and microfluidic perfusion, these organoid models evolve into chip-based human-relevant models capable of parallel drug screens, resistance tracking and personalised therapy prediction. In this review we first outline the principal animal platforms currently guiding breast cancer research, then analyse their successes and limitations, and finally detail how organoids, patient-derived organoid (PDO) biobanks and tumour-on-chip systems can complement or replace animals, accelerating discovery while adhering to the 3Rs principle of refine, reduce and replace.
Why Animal Models Still Dominate, yet Push Us Toward breast cancer organoids organoids as Animal models Alternatives
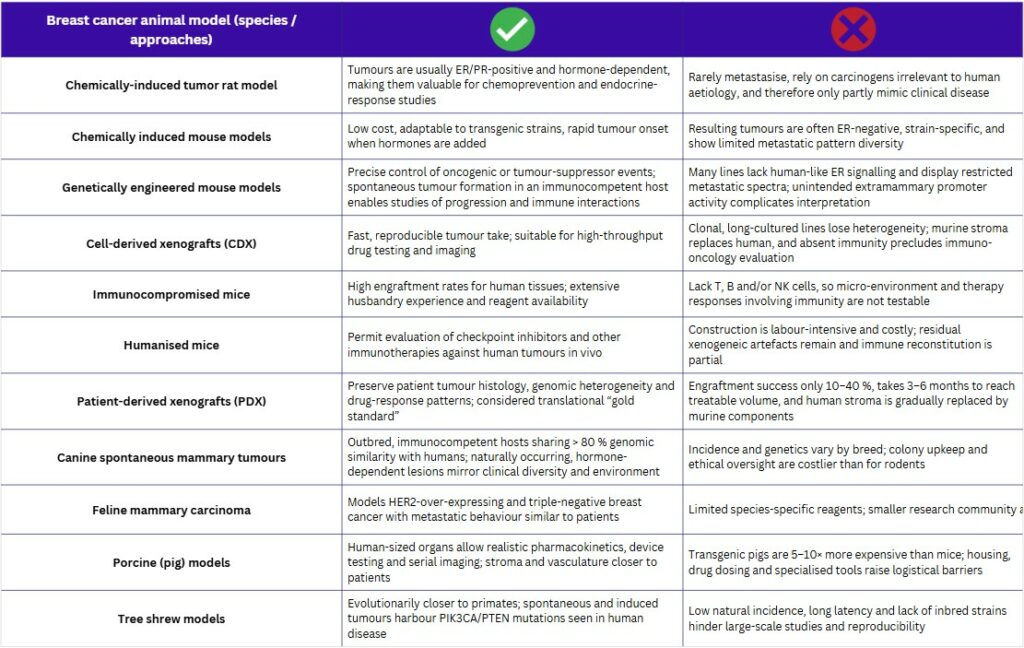
Rodent systems remain the front-line hosts for breast-cancer research. Tumours are provoked with carcinogens such as DMBA or MNU, or induced hormonally, yielding estrogen- and progesterone-responsive lesions in susceptible strains. Parallel genetically engineered mouse models (GEMMs) introduce oncogenic drivers (e.g., MMTV-Neu, Wap-Myc, BRCA1 loss) that generate subtype-specific tumours in immunocompetent animals, enabling longitudinal studies of initiation and metastasis. Nonetheless, murine mammary anatomy, promoter “leakage” and asynchronous latency constrain their fidelity to human disease.
To test therapies, investigators graft human cells into immunocompromised strains such as nude, SCID, NOD-SCID and NSG mice. These hosts boost engraftment but lack key lymphoid compartments, so immune-oncology mechanisms cannot be interrogated. “Humanised” variants partially restore immunity by transplanting human haematopoietic cells, yet each mouse costs several hundred euros and immune reconstitution remains variable, creating logistical and biological hurdles.(1,2)
The translational “gold standard” is the patient-derived xenograft (PDX). Fresh tumour fragments are implanted subcutaneously or orthotopically into these immunodeficient mice, retaining >80 % of the original genomic landscape and faithfully mirroring clinical drug response. Construction, however, is slow (often three to six months) and success rates hover between 10 % and 40 %. Serial passaging gradually replaces human stroma with murine elements, while costs and housing demands limit throughput and real-time clinical utility(3,4).
Larger mammals offer complementary advantages. Canine and feline mammary tumours arise spontaneously, display molecular subtypes analogous to human disease and progress in an intact immune setting, making them attractive for metastasis and immunotherapy studies. Porcine models provide human-sized organs, realistic pharmacokinetics and device-testing opportunities, yet transgenic pigs cost 5–10 × more than mice, require specialised housing and still lack fully validated breast-cancer lines. Limited reagents, small research communities and ethical oversight further restrain large-animal adoption despite their physiological relevance(5).
Collectively, these in-vivo platforms illuminate tumour biology and whole-body pharmacology but are hampered by species gaps, immune artefacts, cost and time. Their persistent shortcomings (evidenced by high attrition of oncology drugs despite promising animal data) now drive the search for breast-cancer organoids as alternative to animal models or patient-derived organoid xenografts and tumour-on-chip systems that bring human relevance, scalability and ethical compliance to pre-clinical discovery.
Learn more about our ready to use breast cancer organoid models.
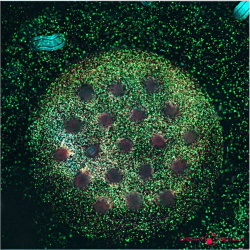
Clinical Attrition and the Translation Gap
Despite an ever-expanding oncology pipeline, only 7.9 % of drug programmes that enter Phase I achieve US-FDA approval, and oncology fares even worse with a mere 5.3 % likelihood of approval (LoA). Exacerbating this low success rate, it now takes an average of 10.5 years for a candidate to travel from first-in-human dosing to market, with oncology Phase I periods (2.7 years) already the longest of any disease area(6).
Multiple factors drive late-stage collapse including patient dropout, under-powered designs and shifting commercial priorities, but analyses of failed trials point first to inadequate translatability of pre-clinical evidence. More than half of Phase III terminations reflect drugs that never delivered meaningful efficacy, while 17 % stumble on safety; both shortcomings often trace back to oversimplified 2D cultures or species-restricted animal studies that mis-represent human tumour biology(7).
Targeted strategies such as patient-preselection biomarkers have begun to lift success odds: programmes that incorporate a genomic or protein marker now show a two-fold higher LoA from Phase I (15.9 % vs 7.6 %) and nearly halve the attrition choke-point at Phase II(6). Yet even with smarter trial design, the industry still depends on discovery platforms that fail to capture the three-dimensional architecture, stromal crosstalk and clonal heterogeneity of human tumours. This gap underscores the urgent need for human-relevant models (notably patient-derived breast cancer organoids as alternative to animal model) that can generate realistic pharmacology and toxicity read-outs before the first patient is enrolled, ultimately shrinking timelines, costs and the persistent chasm of clinical attrition(8).
Regulatory Momentum Driving Advanced In-Vitro Platforms for Breast Cancer Organoid as alternative to animal models
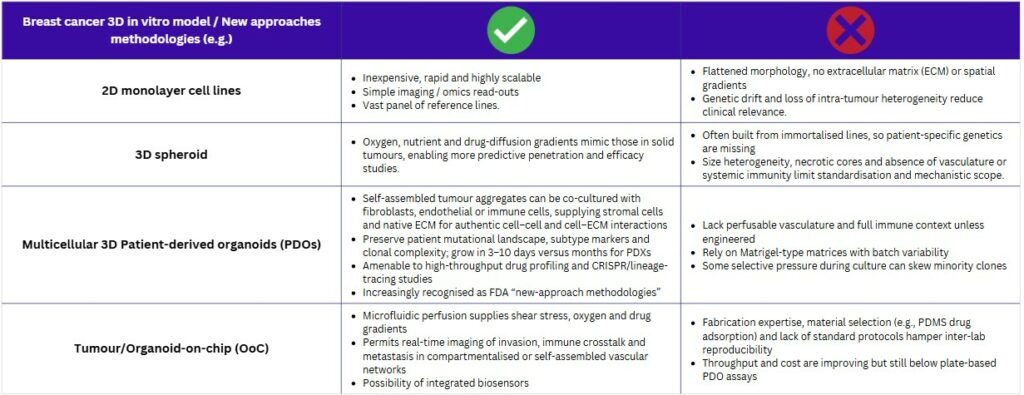
The shift toward human‑centred in‑vitro models is accelerating. A 2014–2019 review logged 935 advanced breast‑cancer test systems (including three‑dimensional cultures, microfluidic chips and other non‑animal methodologies) revealing rapid adoption across laboratories worldwide(9). In parallel, the FDA, EMA and other agencies now champion “new‑approach methodologies” (NAMs) to spare animals and generate data that mirror patient biology, thereby improving late‑stage trial success. Technically, these tools offer clear advantages: PDOs can be grown in days to weeks, whereas PDXs need three to six months, delaying bedside feedback. The quick turnaround, lower cost and genomic fidelity of PDOs make them ideal for precision‑oncology workflows in which treatment decisions cannot wait(4).
To appreciate why regulators value these systems, it is worth defining them. Organoids are self‑organising, three‑dimensional micro‑tissues created from stem or tumour cells embedded in an extracellular‑matrix hydrogel and nurtured with lineage‑specific factors. A tiny biopsy or surgical fragment is minced or enzymatically dispersed; the cells then rebuild epithelium‑like spheres within 3–10 days in vitro. “Breast‑cancer organoid” media supplemented with EGF, Wnt agonists and R‑spondin preserves luminal, basal, HER2 and triple‑negative phenotypes for repeated passage or cryobanking(10).
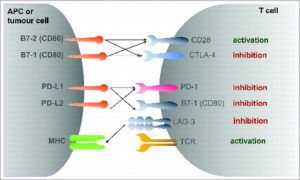
Building on these biological foundations, PDOs now serve the entire oncology pipeline. High‑throughput drug panels rapidly flag subtype‑specific sensitivities and, in compassionate‑use settings, have already redirected adjuvant therapy while the patient is still in clinic(11). Beyond screening, genome editing or barcoding enables mechanistic studies of resistance, whereas co‑culture with autologous immune or stromal cells dissects micro‑environmental interactions(12). Recognising such breadth, regulators increasingly cite organoids as NAMs for pre‑clinical safety testing and have begun integrating them into guidance aimed at reducing animal use.
Across oncology, PDOs are proving their worth as functional read-outs for precision medicine. Brain-tumour PDOs from Peng et al. preserved native immune and stromal ecosystems and predicted individual treatment responses with high accuracy(13), while rectal-cancer PDO chemosensitivity profiles in Ye Yao et al. forecasted chemoradiation outcomes in > 80 % of patients, demonstrating the platform’s predictive power well before radiographic confirmation(14). Breast-cancer data now echo these successes. Mazzucchelli et al. generated matched pre- and post-neoadjuvant PDOs, revealing chemotherapy-driven clonal shifts toward Ki-67-high, stem-like sub-clones and offering a dynamic window on tumour evolution(15). Meanwhile, a living biobank of more than 100 breast-cancer lines compiled by Sachs et al. captured the full spectrum of receptor status, histology and driver mutations; drug screens in this collection paralleled patient responses, underscoring PDOs’ ability to reflect disease heterogeneity and guide precision therapy(16). Collectively, PDOs are high‑fidelity avatars that have the potential to capture evolution, anticipate treatment efficacy and encompass molecular diversity: an advantage PDX models hardly match due to long-term culture. Yet, while they may suffice for some applications, even these miniature tumours lack vascular‑like perfusion and dynamic gradients found in vivo.
Organ‑on‑chip (OoC), and Micro-Physiological System (MPS) technology closes that gap. By marrying microfluidics with 3D culture, OoCs supply continuous nutrients, shear forces and oxygen gradients while permitting real‑time imaging and automated analytics. Specifically breast cancer organoids as an alternative to animal models offer promising outcomes. They therefore deliver animal‑free test beds that more faithfully reproduce tumour micro‑environments and metastatic processes(17,18).
Breast‑specific examples underline the promise. Yang et al’s system perfuses Matrigel domes so that breast‑cancer organoids reach diagnostic size in < 15 days, retain parental histology and generate therapy‑response curves weeks faster than static cultures(19). Complementing this, Testa et al. built a laser‑patterned tumour‑on‑chip whose electrospun scaffold houses cancer and stromal cells in communicating chambers; perfused chemotherapeutics reproduced in‑vivo responses while remaining inexpensive and scalable(20).
Together, these advances show how OoC platforms extend PDO realism with vascular‑like flow and compartmentalised co‑cultures, capturing invasion, immune interplay and therapy response in ways neither static organoids nor animal models can achieve.
Resources
- Animal Models for Studying Prevention and Treatment of Breast Cancer. In: Animal Models for the Study of Human Disease Elsevier; 2013 p. 997‑1018. Disponible sur:
- Zeng L, 中国科学院昆明动物研究所,中国科学院与云南省动物模型与人类疾病机制重点实验室,云南 昆明650223,中国, Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences and Yunnan Province, Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan 650223, China, Li W, Chen CS, 中国科学院大学昆明生命科学学院,云南 昆明650204,中国, et al. Breast cancer animal models and applications. Zool Res. 2020;41(5):477‑94.
- Liu M, Yang X. Patient-derived xenograft models: Current status, challenges, and innovations in cancer research. Genes Dis. sept 2025;12(5):101520.
- Wang E, Xiang K, Zhang Y, Wang XF. Patient-derived organoids (PDOs) and PDO-derived xenografts (PDOXs): New opportunities in establishing faithful pre-clinical cancer models. J Natl Cancer Cent. déc 2022;2(4):263‑76.
- Mondal P, Bailey KL, Cartwright SB, Band V, Carlson MA. Large Animal Models of Breast Cancer. Front Oncol
- BIO, Informa Pharma Intelligence, QLS Advisor. Clinical development success rates and contributing factors 2011-2020
- Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp Clin Trials Commun. sept 2018;11:156‑64.
- Moreno L, Pearson AD. How can attrition rates be reduced in cancer drug discovery? Expert Opin Drug Discov. avr 2013;8(4):363‑8.
- Rossi F, Caforio M, Nic M, Dibusz K, Folgiero V, Romania P, et al. Advanced non-animal models in biomedical research: breast cancer. Luxembourg: Publications Office of the European Union; 2020.
- Huang S, Mei Z, Wan A, Zhao M, Qi X. Application and prospect of organoid technology in breast cancer. Front Immunol. 26 août 2024;15:1413858.
- Thorel L, Perréard M, Florent R, Divoux J, Coffy S, Vincent A, et al. Patient-derived tumor organoids: a new avenue for preclinical research and precision medicine in oncology. Exp Mol Med. 1 juill 2024;56(7):1531‑51.
- Mohan SC, Lee TY, Giuliano AE, Cui X. Current Status of Breast Organoid Models. Front Bioeng Biotechnol
- Peng T, Ma X, Hua W, Wang C, Chu Y, Sun M, et al. Individualized patient tumor organoids faithfully preserve human brain tumor ecosystems and predict patient response to therapy. Cell Stem Cell. févr 2025;S1934590925000025.
- Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell. janv 2020;26(1):17-26.e6.
- Mazzucchelli S, Signati L, Messa L, Franceschini A, Bonizzi A, Castagnoli L, et al. Breast cancer patient-derived organoids for the investigation of patient-specific tumour evolution. Cancer Cell Int
- Sachs N, De Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. janv 2018;172(1‑2):373-386.e10.
- Esposito A, Ferraresi A, Vallino L, Garavaglia B, Dhanasekaran DN, Isidoro C. Three-Dimensional In Vitro Cell Cultures as a Feasible and Promising Alternative to Two-Dimensional and Animal Models in Cancer Research. Int J Biol Sci. 2024;20(13):5293‑311.
- Firatligil-Yildirir B, Yalcin-Ozuysal O, Nonappa. Recent advances in lab-on-a-chip systems for breast cancer metastasis research. Nanoscale Adv. 2023;5(9):2375‑93.
- Yang J, Qu J, Zhang M, Li X, Jiang Q, Kang J, et al. Dynamic culture system advances the applications of breast cancer organoids for precision medicine. Sci Rep. 14 mars 2025;15(1):8852.
- 20. Testa M, Gaggianesi M, D’Accardo C, Porcelli G, Turdo A, Di Marco C, et al. A Novel Tumor on Chip Mimicking the Breast Cancer Microenvironment for Dynamic Drug Screening. Int J Mol Sci. 25 janv 2025;26(3):1028.
FAQ
Rodent systems are frequently employed as the front-line hosts for breast cancer research. Tumours are provoked in susceptible strains using carcinogens, such as DMBA or MNU. Hormones are also used to induce lesions that are responsive to estrogen and progesterone. In parallel, genetically engineered mouse models (GEMMs) are utilized. Oncogenic drivers are introduced in these models. Subtype-specific tumours are generated in animals that have intact immune systems. This permits longitudinal studies of tumour initiation and metastasis. For therapy testing, human cells are grafted into immunocompromised strains, including nude, SCID, or NSG mice. Engraftment is boosted by these hosts.
Several constraints are observed when using mouse models for breast cancer research. The fidelity of these models to human disease is limited by murine mammary anatomy. In genetically engineered mouse models (GEMMs), issues such as promoter "leakage" and asynchronous latency are also found. A separate set of limitations exists for therapy testing. When human cells are grafted into immunocompromised strains (like nude or NSG mice), key lymphoid compartments are absent. Because of this absence, immune-oncology mechanisms cannot be properly interrogated. "Humanised" variants can be used to partially restore immunity. These mice, however, cost several hundred euros each. The reconstitution of the immune system also remains variable. These issues create both logistical and biological hurdles.
The patient-derived xenograft, or PDX, is described as the translational "gold standard". In this system, fresh tumour fragments from a patient are implanted. The implantation is done either subcutaneously or orthotopically into immunodeficient mice. A large amount of the original genomic landscape, reported as over 80 per cent, is retained by the tumour. Clinical drug response is also faithfully mirrored. Despite these positive attributes, several shortcomings exist. The construction of a PDX model is slow. It often requires three to six months. Furthermore, success rates are variable, hovering between 10 and 40 per cent. During serial passaging, the human stroma is gradually replaced with murine elements. High costs and specific housing demands also limit the throughput of these models.
A low success rate is observed for oncology drugs entering clinical trials. This is referred to as the translation gap. Only 5.3 per cent of oncology drug programmes that enter Phase I are eventually approved by the US-FDA. The journey from first-in-human dosing to market takes an average of 10.5 years. A primary reason for these late-stage failures is the inadequate translatability of preclinical evidence. Analyses of failed trials show that more than half of Phase III terminations are due to drugs not delivering meaningful efficacy. Another 17 per cent stumble on safety issues. These shortcomings are often traced back to the models used in discovery. Oversimplified 2D cultures or species-restricted animal studies are noted, as they mis-represent human tumour biology.
A shift toward human-centred in vitro models is being observed. A review logged 935 advanced breast-cancer test systems between 2014 and 2019\. This log revealed rapid adoption across laboratories. In parallel, regulatory agencies such as the FDA and EMA are now supporting "new-approach methodologies," also known as NAMs. These methods are promoted for two reasons. One reason is to spare animals. The other is to generate data that better mirrors patient biology. It is hoped that this will improve the success rates of late-stage trials. Organoids are increasingly cited by regulators as NAMs suitable for pre-clinical safety testing. They have begun to be integrated into official guidance aimed at reducing animal use.
Organoids are defined as self-organising, three-dimensional micro-tissues. They are created from stem cells or tumour cells. The cells are embedded in an extracellular-matrix hydrogel. Lineage-specific factors are used to nurture them. To create one, a tiny biopsy or surgical fragment is minced or enzymatically dispersed. The cells then rebuild epithelium-like spheres. This process occurs in vitro within three to ten days. Specific "breast-cancer organoid" media is used. This media is supplemented with components like EGF, Wnt agonists, and R-spondin. The use of this media preserves the different phenotypes, including luminal, basal, HER2, and triple-negative. The organoids can then be used for repeated passage or for cryobanking.
Patient-derived organoids, or PDOs, are used across the entire oncology pipeline. High-throughput drug panels are run on them. These panels rapidly flag sensitivities that are specific to a tumour subtype. In some compassionate-use settings, PDOs have already been used to redirect adjuvant therapy. This decision was made while the patient was still in the clinic. Beyond simple drug screening, other applications exist. Genome editing or barcoding can be applied to the organoids. This permits mechanistic studies of resistance. Co-culture systems are also being developed. These systems may include autologous immune or stromal cells. Such models are used to dissect micro-environmental interactions.
Yes, patient-derived organoids are being shown as functional read-outs for precision medicine. Brain-tumour PDOs, for example, were reported to have preserved native immune and stromal ecosystems. These models predicted individual treatment responses with high accuracy. In another study, chemosensitivity profiles from rectal-cancer PDOs were used. These profiles forecasted chemoradiation outcomes in over 80 per cent of patients. This demonstration of predictive power was achieved well before radiographic confirmation. Breast-cancer data has echoed these successes. Matched pre- and post-neoadjuvant PDOs were generated, revealing clonal shifts driven by chemotherapy. A living biobank of more than 100 breast-cancer lines was also compiled. Drug screens in this collection paralleled patient responses.
Patient-derived organoids (PDOs) are considered high-fidelity avatars. They have the capacity to capture tumour evolution. Treatment efficacy can also be anticipated. Molecular diversity is encompassed within them. This is a benefit that patient-derived xenograft (PDX) models often do not match because of their long-term culture requirements. Despite these positive attributes, a limitation is noted. Even these miniature tumours are lacking in certain features. They do not have vascular-like perfusion. The dynamic gradients that are found in vivo are also absent from these static cultures. This gap is being addressed by other technologies.
The gap left by static organoids is addressed by organ-on-chip (OoC) technology. This approach marries microfluidics with 3D culture. Continuous nutrients are supplied to the cells. Shear forces and oxygen gradients are also introduced. These systems permit real-time imaging and automated analytics. As a result, animal-free test beds are delivered. These test beds are reported to more faithfully reproduce tumour micro-environments. Metastatic processes can also be modelled. Specific breast-cancer examples show these capabilities. One system perfuses Matrigel domes. This allows breast-cancer organoids to reach diagnostic size in fewer than 15 days, which is weeks faster than static cultures. Another laser-patterned chip houses cancer and stromal cells in communicating chambers.