Over the past decade, three-dimensional “organoid” cultures have evolved from proof-of-concept curiosities into influential tools for human-based drug discovery. Among them, adipose tissue organoids (self-organizing constructs that reproduce the endocrine, metabolic and immuno-stromal features of native fat) have gained interest. These models faithfully secrete adipokines, store and mobilize lipids, and even recruit resident immune cells, thereby capturing aspects of human adipose biology that two-dimensional cultures and rodent studies miss(1).

At a time
when obesity, type 2 diabetes and non-alcoholic steatohepatitis are rising
worldwide, such human-relevant systems promise sharper insights into disease
pathways and therapeutic responses. Regulators are taking notice: recent U.S.
FDA roadmaps and the FDA Modernization Act 2.0/3.0 explicitly encourage
“new-approach methodologies” (NAMs) such as organoids, even as animal studies
remain mandatory for now. By complementing (not yet replacing) traditional
in-vivo tests, adipose-tissue organoids are setting the stage for faster, more
ethical and more predictive preclinical pipelines.
The Importance of Preclinical Testing in Drug Development
Preclinical testing serves as the critical gatekeeper between basic discovery and first-in-human trials. In this phase, investigators must establish a candidate’s pharmacokinetics, target engagement and potential toxicities with enough confidence to justify exposing volunteers or patients to the compound. Historically, this has relied heavily on rodents and, for certain questions, non-human primates. While indispensable for whole-organism toxicology, animal models exhibit pronounced species-specific differences: rodents, for example, possess proportionally more thermogenic brown/beige fat and respond differently to β-adrenergic agonists than humans(2). These disparities contribute to the high attrition rate of metabolism-focused drugs when they transition from bench to bedside.
Emerging human-based systems are beginning to bridge this gap. Organoids, microphysiological “organ-on-chip” platforms, and computational NAMs can
capture human-specific biology earlier in the pipeline, potentially flagging failures before expensive animal studies or Phase I trials. Recognizing this,
the FDA has issued a 2025 roadmap that outlines a stepwise reduction (but not elimination) of mandatory animal tests, contingent on the validation of such
methodologies (3). In this evolving landscape, adipose-tissue organoids occupy a sweet spot: they retain the multicellular complexity needed to model chronic metabolic disease yet can be produced in sufficient quantities for medium-throughput screening. As the field converges on standardized culture protocols and quality-control metrics, these models are poised to make preclinical evaluation both more predictive and more humane.
What Are Adipose Tissue Organoids?
Adipose tissue organoids are three-dimensional, self-organizing constructs that emulate the structural and functional characteristics of human adipose tissue. They are typically derived from primary stromal vascular fraction (SVF) cells or induced pluripotent stem cells (iPSCs) and cultured within extracellular matrix components such as Matrigel or collagen-based hydrogels to promote adipogenic differentiation and tissue organization.
These organoids encompass key cellular constituents of adipose tissue, including mature adipocytes, preadipocytes, endothelial cells, and immune cells like macrophages. Functionally, they exhibit hallmark adipose activities such as lipid uptake and storage, as well as the secretion of adipokines like leptin and adiponectin, which play pivotal roles in regulating energy balance and metabolic processes.
Importantly, adipose tissue organoids can be tailored to replicate different adipose tissue phenotypes, notably white and brown adipose tissues. White adipose tissue (WAT) primarily functions in energy storage, whereas brown adipose tissue (BAT) is specialized for energy expenditure through thermogenesis, facilitated by a high density of mitochondria and the expression of uncoupling protein 1 (UCP1). By manipulating differentiation protocols and culture conditions, researchers can generate organoids that exhibit characteristics of either WAT or BAT, enabling the study of distinct metabolic functions and disease mechanisms associated with each adipose tissue type (4).
This versatility makes adipose tissue organoids a powerful in vitro model for investigating the complex biology of adipose tissues and their roles in metabolic health and disease.
Strategic Advantages of 3D Adipose Organoids and Fat-on-Chip Systems
Three-dimensional adipose-tissue organoids and microphysiological “fat-on-chip” platforms have become central tools in metabolic-disease research by combining the human relevance of primary adipose tissue with the precision, modularity, and automation of modern bioengineering. Unlike conventional 2D adipocyte monolayers, organoids retain the stromal–vascular complexity of native white or brown fat, where mature lipid-laden adipocytes coexist with endothelial cells, progenitor populations, and resident immune cells in an ECM-rich niche. This enables the preservation of key endocrine programs such as leptin and adiponectin secretion, as well as depot-specific transcriptional signatures.
Critically, these platforms offer high experimental versatility: researchers can fine-tune the microenvironment by including or omitting vascular perfusion, endothelial or immune cells, and by selecting scaffolds that mimic specific extracellular matrix properties. Microfluidic fat-on-chip systems further enhance physiological relevance by introducing programmable nutrients and hormone cycles under dynamic flow, simulating shear stress, nutrient delivery, waste removal, and even immune cell trafficking across vascular compartments(5).
Beyond their biological sophistication, these models are engineered for analytical accessibility and scalability. Culture systems are often compatible with imaging techniques and real-time biosensors and designed to support both low- and high-throughput workflows. This accessibility synergizes powerfully with new-generation imaging, in silico modeling, and machine learning approaches, enabling quantitative readouts of complex phenomena such as insulin-stimulated glucose uptake, β-adrenergic lipolysis, and secretion of inflammatory chemokines. Importantly, such platforms allow for parallel evaluation of target-specific efficacy and off-target toxicity in a controlled, human-relevant microenvironment.
Finally, these advances support the 3Rs principles and align with recent regulatory momentum. The 2024 FDA guidance on New Approach Methodologies (NAMs) explicitly acknowledges organoids and organ-on-chip systems as qualified adjuncts, or even partial replacements, for conventional toxicology studies, further accelerating their integration into drug discovery and translational research.
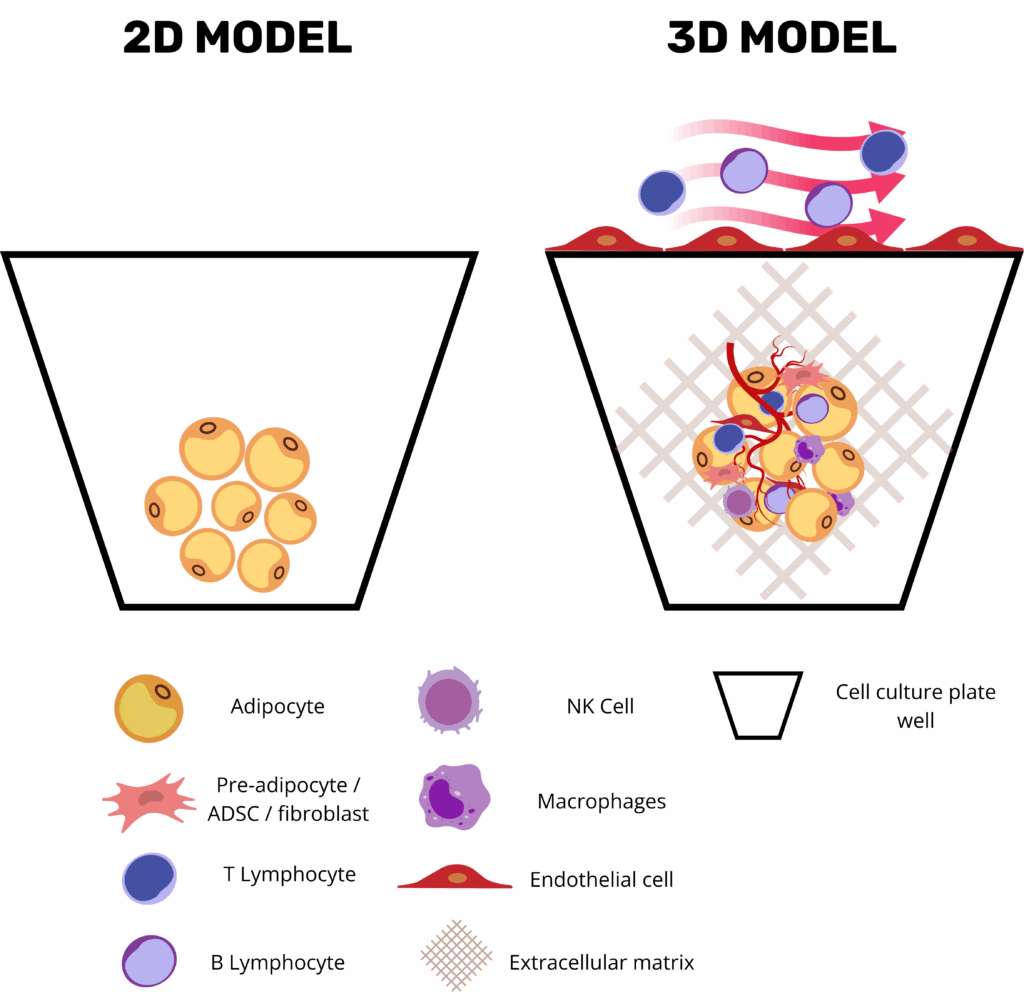
Current Applications of Adipose-Tissue Organoids in Pre-clinical Testing
Adipose-tissue organoids have evolved into a versatile pre-clinical platform that now underpins much of modern metabolic-disease research. By finely tuning the culture environment, investigators can reproduce every major facet of “unhealthy” fat: lipid-droplet hypertrophy, heightened basal lipolysis, a shift in the adiponectin-to-leptin ratio, insulin-signaling defects, and the low-grade inflammatory milieu typical of metabolically diseased adipose tissue(5). Adding pro-inflammatory cytokines or live macrophages deepens that phenotype, generating a chronic, depot-specific inflammation that is ideally suited for evaluating small-molecule or antibody therapies. Because white-adipose organoids (WAOs) can be formed in miniaturized arrays, entire chemical libraries are now screened in parallel; automated imaging and secretome read-outs swiftly flag compounds that normalize lipid storage, temper cytokine release, or restore glucose uptake.
The same three-dimensional scaffolds support co-cultures designed to model insulin resistance. Embedding pre-adipocytes with activated macrophages yields a stable drop in GLUT4 translocation and AKT phosphorylation, giving researchers a human-relevant testbed for PPARγ modulators and next-generation insulin sensitizers(6).
Importantly, when these adipose organoids are grown within biomaterials that mimic the extracellular matrix, such as hydrogels, they also offer a unique opportunity to study how mechanical cues affect fat cell behavior. In this setting, adipocytes respond to stiffness, tension, and pressure by activating mechanotransduction pathways that influence their growth, metabolism, and inflammatory state. These responses reflect how real adipose tissue remodels under stress, such as during obesity or fibrosis, and open the door to testing how mechanical interventions or ECM-targeting drugs could modulate adipose function in disease(7).
Coupling adipose tissue organoids with liver, muscle, or pancreatic modules on microfluidic platforms enables the study of inter-organ crosstalk in metabolic disease. These integrated systems offer insight into how restoring healthy adipose function influences distal tissues, reveal off-target drug effects, and better predict human responses than isolated cell models. For example, in the adipose–liver chip developed by Slaughter et al., hepatocyte steatosis and insulin resistance emerged only in the presence of dysfunctional adipose tissue, and metformin failed to reduce lipid accumulation at clinically relevant doses, highlighting the critical role of adipose-derived signals in NAFLD progression and drug response(8).
Regenerative applications are emerging just as rapidly. Brown- and beige-adipose organoids cultured ex vivo can be implanted beneath the skin or kidney capsule of rodent models; once vascularized, they raise whole-body energy expenditure, blunt weight gain, lower fasting glucose, and, in dyslipidemic animals, bring serum cholesterol and triglycerides within a healthy range. Beyond metabolic regulation, recent work demonstrates that engineered adipose tissue organoids may serve as direct therapeutic agents in cancer, as illustrated by adipose manipulation transplantation (AMT). In this approach, CRISPRa-modified adipose organoids,programmed to upregulate thermogenic and metabolic genes such as UCP1, outcompete tumors for glucose and fatty acids, thereby suppressing tumor growth and progression in both xenograft and genetically engineered cancer models. These findings establish adipose organoids not only as tools for metabolic restoration but also as customizable, cell-based therapeutic platforms for preclinical testing and intervention in cancer(9).
Finally, fat-on-chip platforms are proving useful beyond classical metabolism. Vascularized “omentum” chips that pair adipocytes with mesothelial and endothelial layers allow cancer biologists to dissect how tumor cells exploit adipose niches, while toxicologists use the same devices to gauge how long lipophilic drugs or environmental chemicals persist in human fat and whether they perturb systemic lipid handling. Taken together, these advances show that three-dimensional adipose systems are no longer experimental curiosities; they are already reshaping drug discovery, disease modelling, and early-stage regenerative medicine.
Challenges and Limitations of Adipose Tissue Organoids
Despite their promising applications, adipose tissue organoids face several challenges and limitations that must be addressed to fully realize their potential in preclinical testing.
Complexity of Generation and Maintenance: Creating and sustaining functional adipose tissue organoids require specialized techniques, expertise, and resources. The process involves precise control over the cellular microenvironment to promote proper differentiation and maturation of adipose cells. This complexity can limit the widespread adoption of organoids in research settings, particularly for laboratories with limited access to advanced technologies and training.
Variability and Reproducibility: Organoids derived from different patients or under varying conditions can exhibit differences in structure and function, impacting the reproducibility and reliability of research findings. Standardizing protocols for organoid generation and culture is essential to ensure consistent and comparable results across studies. Additionally, improving the scalability of organoid production is necessary to facilitate large-scale research and drug screening efforts.
Limitations in Replicating Human Physiology: While adipose tissue organoids provide a more accurate representation of adipose tissue than traditional models, they may not fully capture the complexity of systemic interactions and long-term disease progression. Integrating organoids with other tissue models and advanced technologies, such as microfluidic systems and organ-on-a-chip platforms, can help overcome these limitations and enhance the predictive power of organoid-based research(10).
Future Directions in Adipose Tissue Organoid Research
The future of adipose tissue organoid research holds exciting possibilities for advancing our understanding of metabolic diseases and improving therapeutic interventions.
Integration with Advanced Technologies: One promising direction is the integration of organoids with advanced technologies, such as gene editing and high-throughput screening. By leveraging CRISPR/Cas9 and other gene-editing tools, researchers can manipulate specific genes within organoids to study their roles in adipose tissue function and disease. This approach can provide valuable insights into genetic factors influencing obesity, diabetes, and other metabolic disorders, paving the way for targeted therapies.
Development of Multi-Tissue Models: Another area of potential advancement is the development of more sophisticated organoid models that incorporate additional cell types and tissue interactions. For example, creating multi-tissue organoids that include adipose tissue, liver, and muscle cells can provide a more comprehensive understanding of metabolic processes and their systemic effects. These complex models can help elucidate the intricate relationships between different tissues and identify new therapeutic targets for metabolic diseases.
Enhancing Scalability and Reproducibility: Enhancing the scalability and reproducibility of organoid production is also a crucial focus for future research. Developing standardized protocols and automated systems for generating and maintaining organoids can facilitate large-scale studies and drug screening efforts. Additionally, improving the consistency and quality of organoids derived from different patients will enhance the reliability of personalized medicine approaches, enabling more accurate predictions of therapeutic responses and disease outcomes.
Resources
- Liu X, Yang J, Yan Y, Li Q, Huang RL. Unleashing the potential of adipose organoids: A revolutionary approach to combat obesity-related metabolic diseases. Theranostics. 2024;14(5):2075‑98.
- Börgeson E, Boucher J, Hagberg CE. Of mice and men: Pinpointing species differences in adipose tissue biology. Front Cell Dev Biol. 15 sept 2022;10:1003118.
- U.S. Food & Drug Admnistration. Roadmap to Reducing Animal Testing in Preclinical Safety Studies [Internet]. 2025. Disponible sur: https://www.fda.gov/media/186092/download?attachment
- Richard AJ, White U, Elks CM, Stephens JM. Adipose Tissue: Physiology to Metabolic Dysfunction. In: Feingold KR, Ahmed SF, Anawalt B, et al [Internet]. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Disponible sur: https://www.ncbi.nlm.nih.gov/books/NBK555602/
- Contessi Negrini N, Pellegrinelli V, Salem V, Celiz A, Vidal-Puig A. Breaking barriers in obesity research: 3D models of dysfunctional adipose tissue. Trends Biotechnol. mai 2025;43(5):1079‑93.
- Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol-Endocrinol Metab. janv 2007;292(1):E166‑74.
- Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. févr 2022;185(3):419‑46.
- Slaughter VL, Rumsey JW, Boone R, Malik D, Cai Y, Sriram NN, et al. Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical therapeutic efficacy evaluation. Sci Rep. 23 juin 2021;11(1):13159.
- Nguyen HP, An K, Ito Y, Kharbikar BN, Sheng R, Paredes B, et al. Implantation of engineered adipocytes suppresses tumor progression in cancer models. Nat Biotechnol [Internet]. 4 févr 2025 [cité 4 avr 2025]; Disponible sur: https://www.nature.com/articles/s41587-024-02551-2
- McCarthy M, Brown T, Alarcon A, Williams C, Wu X, Abbott RD, et al. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng Part B Rev. 1 déc 2020;26(6):586‑95.