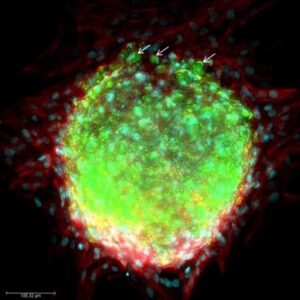
The tumor microenvironment (TME) is a complex biochemical network that includes multiple factors surrounding tumor cells, such as vascular networks, the extracellular matrix (ECM), and a heterogeneous cell population composed of multiple cell types like fibroblast or immune cells.
This Scientific Note briefly describes the main components of the TME and their interactions, together with their role in tumor growth and tumor evolution.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Components of the tumor microenvironment
In living tissues, cells respond to multiple physical and biochemical cues which are established by the local cellular microenvironment [1]. Inside a tumor, tumor cells are surrounded by a highly modified stroma known as the tumor microenvironment or TME. In this microenvironment, cell-cell interactions, cell-extracellular matrix interactions, and factors released by both, cancer and stromal cells, are critical for tumor growth and development. Through these complex interactions, tumor microenvironment can affect gene expression, cell metabolism, chromatin structure, cell proliferation and cell migration [2]. The following video summarizes some interactions in the TME.
The main components of the TME are: vascular networks, the extracellular matrix, and cells.
Vascular Networks: The formation of a highly vascularized environment facilitates tumor progression by providing nutrients and additional factors secreted in the tumor microenvironment. Tumor angiogenesis occurs through the secretion of VEGF (Vascular Endothelial Growth Factor, see below), by cancer cells, fibroblasts, and stromal immune cells. This vascularization process surrounding tumors is abnormal regarding its arrangement and vessel composition. Vessels are leaky, which generates high interstitial pressures and an accumulation of molecules in the bloodstream [4]. Additionally, vascularization contributes to hypoxia. The link between abnormal vascular networks and hypoxia in the tumor microenvironment is further described in the Scientific Note Cancer and Hypoxia.
The Extracellular Matrix (ECM) is composed of extracellular proteins and proteoglycans, like collagen, laminin, elastin, fibrinogen, fibronectin, and vitronectin. The following video summarizes the function and composition of the ECM in healthy tissues:
In tumor cells, the ECM is highly remodeled through extracellular factors released by different cell types in the TME, including cancer cells. In the TME, cell contacts with the extracellular matrix activate signaling pathways like anti-apoptotic pathways. Interactions of cancer cells with the ECM can induce drug resistance in a process termed CAM-DR (cell adhesion mediated drug resistance) [3] [5]. Some extracellular signaling factors released in the ECM are (reviewed in [4]):
-TGF-β (Transforming Growth Factor β): In normal tissues TGF- β is a growth inhibitor, regulating tissue homeostasis and apoptosis and an immune system suppressor. However, TGF-β has proliferative effects on cancer cells and plays a key role in the epithelial to mesenchymal transition (EMT) (see below).
-SDF-1 (stromal cell-derived factor): A gradient of SDF-1 secreted by stromal cells is generated in the ECM and it contributes to the recruitment of cancer cells expressing a receptor for it, through chemotaxis. Ligand-receptor interactions induce cell migration and proliferation.
-HIF: It is a family of transcription factors activated upon hypoxia, which are involved in metabolism, angiogenesis, proliferation, and ECM remodeling in response to hypoxia [5].
-VEGF (Vascular Endothelial Growth Factor). It is mainly secreted by fibroblasts (CAFs, cancer-associated fibroblasts), but also cancer cells and immune cells. As a vascular growth factor, it is a major contributor of the abnormal angiogenesis occurring at the TME. It has also a role in extracellular matrix remodeling.
-SPARC (secreted protein acidic rich in cysteine), or osteonectin. Secreted in the stroma by fibroblasts and together with a role in proliferation and angiogenesis, it has a direct function on remodeling the extracellular matrix [4].
-Matrix Metallo Proteinases (MMPs): Secreted by fibroblasts and other stromal cell types, they have a function in hydrolysis of proteins in the extracellular matrix, like collagen or laminin, or cleavage of cytokines. This direct modification of the ECM has an impact on the ability of cancer cells to invade their surroundings, by disrupting cell-ECM interactions. They are also involved in the epithelial to mesenchymal transition ETM (see below). Because of the important role of MMP molecules in cancer progression, they have been targets of anti-cancer therapies and mouse models of MMPs have been developed [4][5]
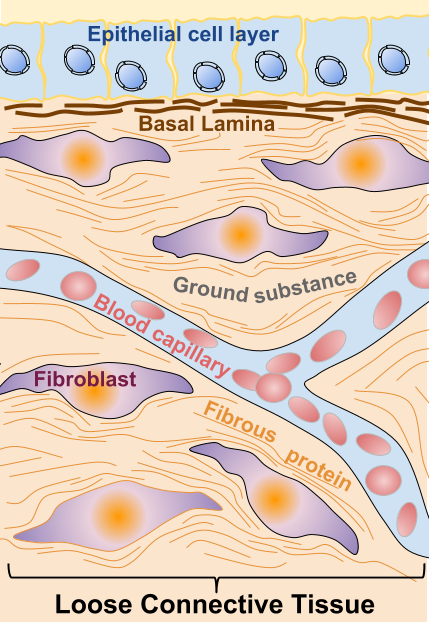
Cell types in the TME
The most abundant cell types present in the TME with a role in tumor development, which participate in the secretion of the extracellular factors previously described are:
Fibroblasts: They are the most abundant cell type in the TME and they can be even more numerous than cancer cells [4]. Fibroblasts are modified during tumor progression, being remodeled into CAFs: carcinoma-associated fibroblasts. CAFs are functionally active, and as opposed to functionally active normal fibroblasts (active for instance in wound healing), CAFs are constitutively active and they don’t undergo apoptosis. Also, CAFs cannot revert to normal fibroblast. Mechanisms converting normal fibroblasts into CAFs in the stroma are not completely understood [4]. Surrounding cells, like tumor cells, cells undergoing an epithelial to mesenchymal transition (EMT) or bone marrow-derived cells (BMDCs), have been shown to be possible origins of CAFs [4]. Activated CAFs have been shown to have a role in cancer initiation and as well as enhancing metastasis.
Epithelial cells: Epithelial cells in the TME can undergo the EMT (epithelial to mesenchymal transition) is a process through which cells loose contacts (cell to cell and cell-ECM contacts) and acquire motility and invasion capacity, allowing them to migrate from the primary tumor site [5].
Mesenchymal stem cells: MSC is progenitor pluripotent cells type cells, bone marrow-derived, which are recruited to the TME and are modified into CA-MSC (carcinoma-associated MSC), losing their pluripotency and contributing to tumor growth, angiogenesis, and metastasis [2].
Immune cells: Different types of immune cells are present in the tumor microenvironment and are known as IICs (infiltrating immune cells). Macrophages are recruited to the cancer location and become activated as TAMs (Tumor-associated macrophages). TAMs are recruited in hypoxic zones and release a number of factors that contribute to tumor development. They upregulate transcription factors or participate in the activation of endothelial cells. Neutrophiles can induce angiogenic processes through the release of VEGF (Vascular Endothelial Growth Factor), and leukocytes generate gradients of secreted growth factors that induce tumor movement towards tumor vasculature [2] [4][6].
The impact of the TME on tumor development depends on complex interactions of the above described extracellular factors and cell types. Anti-cancer therapies targeting different components of the TME (and particularly the ECM) have been developed. Targeting the TME is complex since stromal cells and ECM components are not unique to the tumor microenvironment and these therapies should target only modified phenotypes. Also, drug delivery methods are highly dependent on the TME [4]. Anti-angiogenic drugs (mainly targeting VEGF) are an example of anti-cancer drugs directly targeting the TME used in combination with other drugs.
References
[1] Chen C.S. 3D Biomimetic Cultures: The Next Platform for Cell Biology. Trends in Cell Biology (2016). https://www.ncbi.nlm.nih.gov/pubmed/27637342
[2] Castells M. et al. Implication of Tumor Microenvironment in Chemoresistance: Tumor-Associated Stromal Cells Protect Tumor Cells from Cell Death. Int. J. Mol. Sci. 2012 https://www.ncbi.nlm.nih.gov/pubmed/22949815
[3] Jo Y. et al. Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-mimicking In Vitro Models in Anti-Cancer Drug Development. Theranostics. 2018. https://www.ncbi.nlm.nih.gov/pubmed/30555545
[4] Li HC et al. Tumor microenvironment: The role of the tumor stroma in cancer. J Cell Biochem. 2007 https://www.ncbi.nlm.nih.gov/pubmed/17226777
[5] Finger EC. and Giaccia AJ. Cancer Metastasis Rev. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease (2010) https://www.ncbi.nlm.nih.gov/pubmed/20393783
[6] Hanahan D. and Coussens LM. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment”. Cancer Cell (2012) https://www.ncbi.nlm.nih.gov/pubmed/22439926
Videos:
https://www.youtube.com/watch?v=U5ccW209BYY
https://www.youtube.com/watch?v=cMNx17H3dRU