The Liver: Overview, Histology and Pathology
The liver (Fig. 1) is the largest organ in the human body and responsible for 1stand 2ndpass metabolism, where it is the nexus for nutritional and drug metabolism as well playing a crucial role in the excretory system for removal of endogenous waste products or xenobiological compounds. Optimal liver functioning requires temperature homeostasis (normothermia) and oxygen supply (normoxia) in this high metabolic demand organ to ensure optimal activity of all the endogenous cells as well as intra-organ biochemical communication. The bulk of the cell types found in liver tissues are mainly hepatocytes, with liver-specific immune cells called Kupffer cells, stellate cells, liver sinusoidal endothelial cells, and cholangiocytes [ref]. Figure 1 shows selected liver tissue-associated cells. Liver Biopsies
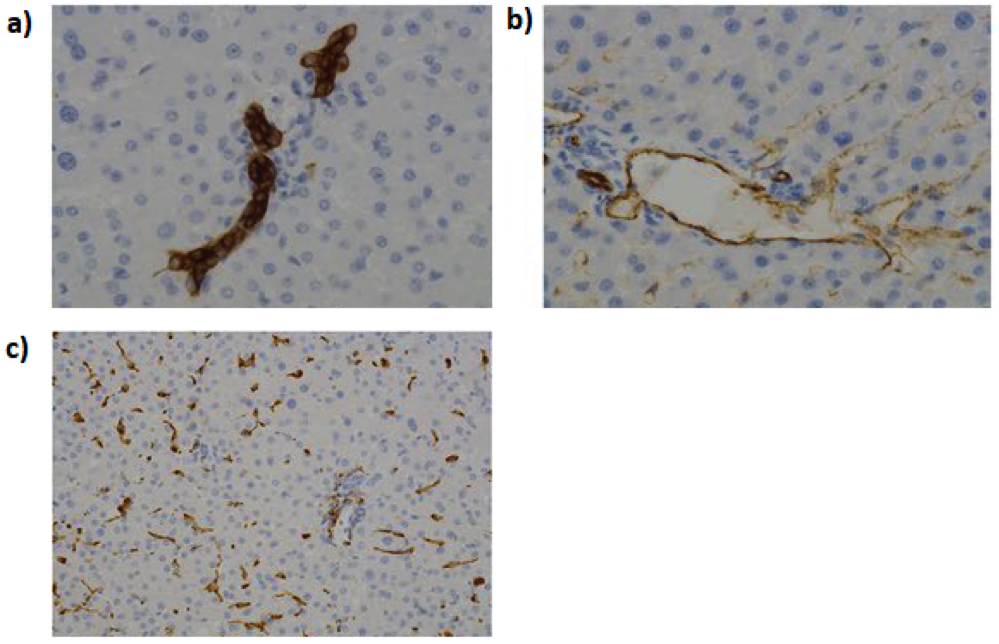
Figure 1: Histological analysis of human liver. Cells were stained for improved contrasting, where the biliary epithelial cells (a); endothelial cells (b); and macrophages are readily identifiable from the main tissue mass. (Figure taken and adapted from Paish et al, 2019).
Liver disease and associated pathologies are mainly attributed (±80%) to lifestyle and environmental factors, resulting in ±2 million deaths globally. Cirrhosis and liver cancer (hepatocellular carcinoma) combined accounts for 3.5% of all global mortality with the former ranked 11thand the latter ranked 16thcause of death, respectively. Global alcohol consumption puts 2 million people at risk for alcohol associated liver disease, where 2 million more adults are obese/overweight and there are 400 million adults with diabetes, both high risk factors for fatty liver disease and/or cancer. Additionally, viral hepatitis infections globally remain high which can lead to new cases of cirrhosis, cancer or fatty liver disease. Liver transplants, the 2ndmost transplanted organ, occur ±20200 globally per annum [ref] which equates to a 10% matching of needs. Roughly 15% of these transplants are done using partial liver donation from living patients.
The liver and associated research models is seen as integral and crucial for pharmacological applications relating to absorption-distribution-metabolism-excretion-toxicology (ADMET) as well as the mechanistic studies of pathology initiation and progression.
Liver Biopsy – Clinical Applications in Humans and Animals
Liver-associated biomarkers for physiological and biochemical events can be found primarily in the blood and urine, enabling the diagnosis of various pathological disease states. However, a liver biopsy and histological analysis provides a definitive diagnosis and can assist in prognosis. The biopsy is done using percutaneous, laparoscopic or transvenous methods, typically guided using ultrasound, magnetic resonance imaging (MRI) or computed tomography (CT) approaches as guidance. Biopsies are essential for the following types of liver disease (Fig. 2):
- Drug induced liver-injury [ref] or liver sensitivity to chemotherapy;
- Non-alcoholic steatohepatitis (NASH) or alcohol induced cirrhosis;
- Viral (e.g. Hepatitis) [ref] or zoonotic infection;
- Viability of liver tissue for a transplant as well as post-transplant analysis;
- Hepatic cancer, primary as well as metastatic; and
- Post-mortem cause of death investigation.

Figure 2: Comparative histology for a normal liver (a) and livers presenting with various pathologies such as NASH (b), steatosis (c) and cirrhosis (d). (Taken and adapted from Mezale et al, 2017)
Veterinary applications of liver biopsies, in additional to similar uses as for humans, allows for facile determination of various physiological and pathological disease states in livestock, where liver biopsies allow for the determination of systemic copper accumulation, nutritional status, environmental toxicity and liver fat content.
Liver Biopsy – Beyond Histology towards Precision Patient Specific Pharmacology and Disease Investigation
Precision cut slices (PCS) of liver biopsy tissue have enabled the study of liver tissues ex vivo, preserving tissue complexity with regards to cell types and ratios as well as providing an intact and native extracellular matrix. Adoption of PCS methodologies in hepatology provides advantages over using 2D monocultures:
- Hepatocytes lose many phenotypic properties 24-48h, especially for expression of cytochrome p450 enzyme;
- Hepatocytes in a 2D culture are hypersensitized to chemotherapeutic compounds;
- There is no immune system interactions or other cell-to-cell / organ-to-organ communication; and
- Absence of biomechanical stimulation and interaction with an extracellular matrix (ECM).
Liver biopsy derived PCS, from healthy and diseased livers, have been used (Fig. 3) to provide precision pharmacology and therapeutic intervention in real-time for the individual patient (biopsy-on-chip) or to generate patient-derived cells for fabricating in vitro models (e.g. organoids, liver pathology on-chip, bioprinting) approaches for personalized drug response and ADMET studies. Patient derived liver biopsy cells have also been used to create spheroid/organoid biobanks, where the genotype-phenotype relationship of the pathology has been well preserved, advancing drug discovery / development and ADMET approaches. The advantages of using liver biopsy for translational as well as basic research provides advantages over cell lines (immortalized, primary and stem cells) and 3D engineered tissues. Peer reviewed papers have been published for various pathologies where drug response, immune cell interaction as well as inducing pathologies in healthy tissues.

Figure 3 : The uses of liver biopsy tissues from histology to in vivo/ in vitroculturing methodologies. Precision cut slices (PCS) can be directly cultured, used to generate in vivo xenograft models, patient derived in vitro cultures as well as for tissue engineering approaches such as liver-on-chip and bioprinting. Various research methodologies have been developed to utilize cells/tissues to develop derived models (blue arrows). Stem cells and immune cells can also be isolated from patient derived tissues, allowing for a more complex in vitro model for mechanistic research.
Incubator dependent static liquid culturing, with and without rocking platforms) of liver biopsy or patient derived liver tissue (PCL) in static culturing has the same challenges as for many other in vitro methodologies:
- Loss of cellular phenotypes and overall tissue integrity within 24-72h;
- Tissue necrosis due to hypoxia attributed to poor oxygen diffusion (loss of normoxia) in liquid culture and oxygen gradients in commercial CO2-incubators;
- Non-physiological waste accumulation in static culture media; and
- Temperature shocks (hypothermia) with removal from 37°C-CO2incubators to biocabinets (±20-25°C) – a loss of normothermia.
Reports of using bioreactor systems with perfusion addressed most of these challenges, allowing for disease phenotype and overall biochemistry to be near-physiological for up to 7 days, with a total of 14 days of continuous culturing. Unfortunately, current commercially available perfusion systems are expensive and require companion technology to assure regulation of temperature and gas diffusion. The current limitations in direct biopsy derived PCL culturing require researchers to forego tissue complexity and utilize single/multi cell derived cultures (e.g. spheroids/organoids) or in vivo methodologies, utilizing static culturing for the former and low grafting success as well as expensive for the latter.
PCL culturing of liver biopsies have potential to enable patient specific pharmacologic solutions, but the technology and methodology is currently only achievable for well-funded research groups and clinicians. Providing an accessible technological solution for direct liver PCL culturing to address normoxia, normothermia and provide physiological mimicry for nutrient supply / waste removal can open the doorway to more robust and standardized research for precision pharmacology as well as drug discovery.
Discover our liver project !
References
Allen, J.W. and Bhatia, S.N. 2002. Formation of Steady-State Oxygen Gradient In Vitro. Biotechnology and Bioengineering, 92(3):253-262.
Blumer, T. 2019. Hepatocellular Carcinoma Xenografts Established from Needle Biopsies Preserve the Characteristics of the Originating Tumors. Hepatology Communications, 3(7):971-986.
Boyd, A., et al. 2018. Medical Liver Biopsy : Background, Indications, Procedure and Histopathology. Frontline Gastroenterology, 0:1-8.
Byass, P. 2014. The Global Burden of Liver Disease: A Challenge for Methods and for Public Health. BMC Medicine, 12:159.
Calabrese, D., et al. 2019. Liver Biopsy Derived Induced Pluripotent Stem Cells Provide Unlimited Supply for the Generation of Hepatocyte-Like Cells.PLOSOne, https://doi.org/10.1371/journal.pone.0221762.
Hadi, M., et al. 2013. Human Precision-Cut Liver Slices as an Ex Vivo Model to Study Idiosyncratic Drug-Induced Liver Injury. Chem, Res, Toxicol., 26(5):710-720.
Kleiner, D.E. 2019. Liver Histology in the Diagnosis and Prognosis of Drug-Induced Liver Injury. Clinical Liver Disease, 4(1):12-16.
McCarron, S. 2019. NASH Patient Liver Derived Organoids Exhibit Patient Specific NASH Phenotypes and Drug Responses. bioRXiv, doi:https://doi.org/10.1101/791467.
Mezale, D., et al. 2017. Chapter 1: Non-Alcoholic Steatohepatitis, Liver Cirrhosis and Hepatocellular Carcinoma: The Molecular Pathways, InTech, http://dx.doi.org/10.5772/intechopen.68771.
Nostedt, J.J. et al. 2018. Normothermic Ex Vivo Machine Perfusion for Liver Grafts Recovered from Donors after Circulatory Death : A Systematic Review Meta-Analysis. HPB Surgery, https://doi.org/10.1155/2018/6867986.
Nucifero, S., et al. Organoid Models of Human Liver Cancers Derived from Tumour Needle Biopsies. Cell Reports, 24:1363-1376.
Olinga, P. and Schuppan, D. 2013. Precison-Cut Liver Slices: A Tool to Model the Liver Ex Vivo. Journal of Hepatology, 58:1252-1253.
Op den Dries, S., et al. 2013. Ex Vivo Normothermic Machine Perfusion and viability Testing of Discarded Human Donor Livers. Am. J. of Transplantation, 13:1327-1335.
Paish, H.L., et al. 2019. A Bioreactor Technology for Modeling Fibrosis in Human and Rodent Precision-Cut Liver Slices. HEPATOLOGY, 70(4):1377-1391.
Palma, E., et al. 2019. Precison-Cut Liver Slices : A versatile Tool to Advance Liver Research. Hepatology International, 13:51-57.
Piera, T., et al. 2016. Organ Culture Model Of Liver for the Study of Cancer Treatment for Hepatocellular Carcinoma. Cancer Research Journal, 4(2):37-42.
Rocky, D.C., et al. 2009. Liver Biopsy. HEPATOLOGY, doi:10.1002/hep.22742.
Taltavull, T.C. Chapter 3: Rethinking the Role of Liver Biopsy in the Era of Personalized Medicine. InTech, http://dx.doi.org/10.5772/53120.
Tanon, F., et al. In Vitro Metabolic Zonation Through Oxygen Gradient on a Chip. Scientific Reports, 9:13557.
Tostoes, R.M., et al. 2012. Human Liver Cell Spheroids in Extended Perfusion Bioreactor Culture for Repeated-Dose Drug Testing. HEPATOLOGY, 55(4):1227-1236.
Wu, X. 2018. Precision-Cut Human Liver Slice Cultures as an Immunological Platform. J. Immunol Methods, 455:71-79.
Yu, F.m et al. 2017. A Perfusion Incubator Liver Chip for 3D Cell Culture with Application on Chronic Hepatoxicity Testing. Scientific Reports, 7:14528.


