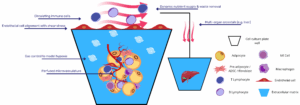
Obesity, type 2 diabetes, and non-alcoholic fatty liver disease (NAFLD) remain major global health concerns, yet preclinical models continue to rely heavily on rodent adipose tissue, despite well-documented interspecies differences in depot structure, immune composition, and adrenergic responsiveness. While three-dimensional adipose tissue organoids have improved physiological relevance compared to two-dimensional cultures, their static nature imposes limitations in oxygen and nutrient diffusion, mechanical stimulation, and real-time metabolic monitoring. In recent years, adipose tissue on chip platforms have emerged as a promising microphysiological alternative, offering a dynamic environment in which human adipocytes can be studied under controlled perfusion and physiological flow.
These systems integrate mature or stem cell-derived adipocytes within microfluidic devices that support co-culture with endothelial and immune cells, enabling real-time analysis of endocrine signaling, lipid metabolism, and inflammatory responses. Various approaches have demonstrated the ability to recapitulate insulin resistance, lipotoxicity, cytokine release, and cross-tissue metabolic interactions, including adipose–liver crosstalk in NAFLD models. As shown in recent studies, the incorporation of vascular compartments, extracellular matrix tuning, and immunocompetent niches further improves the functional maturity and disease relevance of adipose tissue chips.
Positioned within the growing category of NAMs and supported by regulatory guidance from the U.S. FDA and OECD, adipose tissue-on-chip systems are gaining traction as predictive, human-relevant models for therapeutic screening and mechanistic discovery. This article reviews the development and application of adipose-on-chip technologies, focusing on design strategies, physiological modeling, disease-specific adaptations, and integration within multi-organ platforms.
1 │ From Static Spheroids to Dynamic Adipose Tissue on Chip Platforms
Three-dimensional adipose organoids marked a decisive step beyond monolayer culture, yet their static configuration still confronts two fundamental constraints. First, diffusion limits: in scaffold-free or hydrogel spheroids O₂ and nutrient gradients develop rapidly, producing lipid-poor multilocular adipocytes and compromising long-term endocrine read-outs. Reviews of dysfunctional-fat models identify this gradient as a primary reason unilocular white-fat morphology and stable adiponectin secretion are rarely maintained beyond 2–3 weeks(1). Second, the absence of mechanical and fluid cues: without interstitial flow, adipocytes never experience the shear, convective transport or continual endocrine clearance that define in-vivo depots.
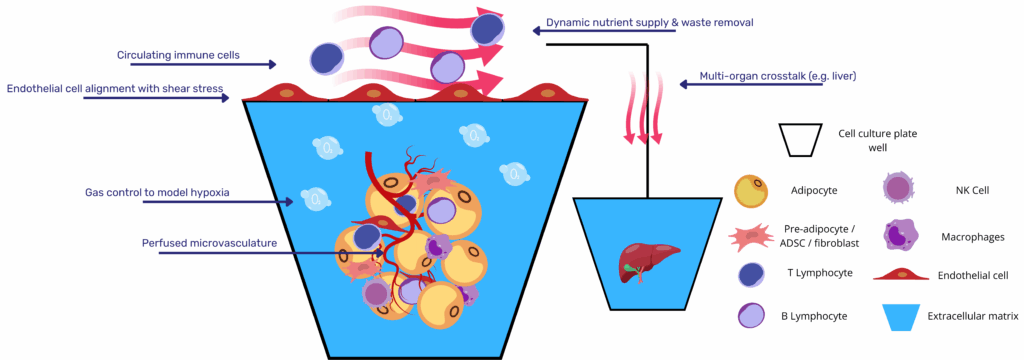
Microfluidic fat-on-chip systems tackle both shortcomings by housing adipocytes in perfused chambers that are hydraulically isolated from damaging shear yet continuously supplied with medium. Liu and co-workers demonstrated that a laminar flow of 8 nL s⁻¹ across a three-compartment device supported weeks-long culture of differentiating human pre-adipocytes, with lipid droplets expanding under constant nutrient exchange and waste removal(2). Rogal’s “WAT-on-a-chip” extended the concept to mature primary adipocytes, integrating shielding side channels so buoyant cells remain viable while a vascular-like conduit enables online monitoring of fatty-acid uptake and isoproterenol-induced lipolysis(3).
Dynamic perfusion further unlocks immunometabolic investigation. In Kongsuphol’s inflamed-adipose model, co-culturing peripheral-blood mononuclear cells with on-chip adipocytes produced time-resolved cytokine waves (IL-6, TNF-α) and a concomitant drop in insulin-stimulated glucose uptake; features difficult to capture in static transwells(4). Huff et al. refined vascular integration, embedding endothelial cells across a 3 µm porous membrane; the resulting endothelial barrier controlled molecular diffusion and preserved >90 % cell viability under dual-flow conditions while allowing non-invasive sampling of triglycerides and resazurin metabolism(5).
Beyond single-tissue fidelity, perfused adipose chips are readily networked with additional organs. Slaughter’s adipose–liver circuit showed that free-fatty-acid–rich effluent from hypertrophic fat exacerbates hepatocyte steatosis and alters CYP3A4 activity, inter-organ effects unobservable in isolated cultures(6). Such studies position the adipose chip squarely within the expanding repertoire of MPS recognized by regulators as NAMs for metabolic disease research.
Collectively, evidence from first-generation platforms indicates that transitioning from static organoids to perfused adipose tissue on chip devices yields more mature lipid architecture, real-time endocrine profiling and mechanistic access to immune and multi-organ crosstalk, capabilities essential for predictive drug discovery in obesity, diabetes and NAFLD pipelines.
Learn more about our ready to use Adipose organoid plate.
2 │ Cells, Channels & Matrices: Core Design Principles of the Adipose Tissue on Chip
Successful microphysiological modelling of human adipose tissue begins with the cellular constituency. Early devices relied on pre-adipocyte lines differentiated on-chip, yet lipid accumulation and endocrine output remained modest. Subsequent studies turned to patient-derived stromal-vascular fractions (SVF) or purified mature adipocytes. Rogal et al. showed that buoyant primary adipocytes can be stabilized inside shallow micro-cavities, preserving unilocular morphology and leptin secretion for three weeks under flow(3). Huff et al. achieved comparable stability with SVF-derived adipocytes differentiated within hyaluronic-acid hydrogels, reporting >90 % viability and robust triglyceride turnover after 28 days(5). For brown-fat applications, ADSC or hiPSC progenitors seeded on-chip can be thermogenically driven to produce UCP1‐positive constructs that oxidize fatty acids at rates unattainable in static cultures.
Vascular architecture is the second pillar. Two strategies dominate. The parallel-channel design places an endothelialized micro-channel beside the adipose chamber; nutrients and cytokines diffuse across a porous membrane while adipocytes remain shielded from damaging shear (<0.05 dyn cm⁻²). The self-assembled niche embeds endothelial cells directly within the matrix; spontaneous sprouting yields capillary-like meshes that infiltrate spheroids, supporting hypertrophy and improving insulin responsiveness, as demonstrated by Baganha et al.(7). Both geometries permit continuous sampling of effluent for adipokines, glycerol and non-esterified fatty acids.
Matrix selection modulates depot identity. Soft (≈2 kPa) collagen- or HA-based hydrogels encourage formation of large unilocular droplets characteristic of white adipose tissue, whereas stiffer GelMA or PEG-RGD formulations bias differentiation toward beige/brown phenotypes via YAP-mediated mechanosignalling(1). Decellularized adipose ECM offers compositional fidelity but introduces donor variability; photo-tunable synthetic matrices now allow stiffness ramps during culture to mimic progressive fibrosis.
Integrated sensing closes the loop between structure and function. Huff’s platform incorporates oxygen and pH micro-electrodes in flow channels, correlating hypoxic shifts with bursts of IL-6 during lipotoxic challenges(5). Rogal couples resazurin reduction, glucose amperometry and automated microscopy, enabling real-time calculation of lipolytic rate constants during β-adrenergic stimulation(3).
Inflammation is a principal driver of metabolic dysfunction, and recent designs therefore add an immune compartment. Kongsuphol et al. perfused peripheral-blood mononuclear cells through an adipose chamber; migrating macrophages elicited TNF-α and IL-6 surges and reduced insulin-stimulated glucose uptake, reproducing early events of obesity-linked insulin resistance(4). Similar approaches seed resident SVF macrophages or mast cells directly into the matrix, preserving an M2-skewed phenotype under homeostatic flow and enabling controlled polarization to M1 for chronic-inflammation studies.
Finally, modularity facilitates cross-tissue interrogation. Adipose chambers have been perfused in series with liver spheroids to model NAFLD, with hepatocyte steatosis scaling to free-fatty-acid flux from upstream fat tissue(6). Coupling to tumor models tracks lipid transfer that fuels invasion, while pancreas-on-chip integration aims to capture adipose-driven β-cell stress. With tunable cell sources, engineered vasculature, mechano-responsive matrices and immune inclusivity, the adipose tissue on chip provides a versatile foundation for predictive studies of metabolic disease and therapeutic screening.
3 │ Disease Modelling and Drug Discovery with Perfused Adipose Tissue on Chip
Microfluidic adipose platforms increasingly serve as experimental “disease depots,” allowing pathologies to be induced and monitored under precisely controlled biochemical and mechanical conditions. Inflammatory insulin resistance is a primary use-case. In the inflamed-adipose model of Kongsuphol et al., peripheral-blood mononuclear cells perfused through SVF-derived adipocytes triggered ten-fold surges in TNF-α and IL-6 and reduced insulin-stimulated glucose uptake by 60 %, faithfully mirroring early obesity-linked dysfunction(4). Liu et al. layered cyclic high-glucose pulses onto a similar chip; the combination of immune flow and glucotoxic stress produced sustained serine phosphorylation of IRS-1, an effect absent in static spheroids(2).
Hypertrophic growth, another hallmark of visceral obesity, has been reproduced through matrix engineering and long-term perfusion. Huff et al. tuned hyaluronic-acid stiffness to 2 kPa and applied low-shear flow for 28 days; adipocytes expanded to a mean diameter of 110 µm, secreted leptin at > 30 ng mL-¹ day-¹ and displayed elevated basal lipolysis, closely matching clinical observations(5). Leung et al. achieved comparable hypertrophy without exogenous cytokines by perfusing palmitate/oleate through an HA scaffold; IL-6 rose four-fold and lipid-droplet area doubled, providing a physiologically driven obesity model suited to downstream muscle or liver coupling(8). Rogal’s WAT-on-a-chip, using primary mature adipocytes, coupled real-time resazurin reduction and glycerol amperometry to quantify metabolic-rate shifts during hypertrophy(3).
Perfused adipose modules also enable inter-organ crosstalk studies. In a two-tissue circuit, Slaughter et al. placed a hypertrophic adipose tissue on chip upstream of primary human hepatocytes; free-fatty-acid and cytokine flux from the fat module increased hepatocyte triglyceride accumulation by 45 % and suppressed CYP3A4 activity, modelling the transition from simple steatosis to inflammatory NAFLD(6). Similar topologies couple vascularized adipose tissue to ovarian-cancer chips; sustained lipid delivery fuels tumor invasion and chemoresistance, underscoring the systemic reach of dysfunctional fat.
Drug-screening applications illustrate the translational value of these disease analogues. On Kongsuphol’s inflamed chip, the dual PPARα/δ agonist elafibranor normalised IL-6 secretion and restored > 80 % of insulin sensitivity at 10 µM, whereas identical dosing in static transwells showed only marginal benefit(4). Huff’s platform enabled non-invasive mitochondrial stress testing; rosiglitazone reversed palmitate-induced reductions in spare-respiratory capacity within 48 h, an effect captured via on-chip oxygen electrodes(5). Rogal demonstrated that isoproterenol-stimulated lipolysis curves shift rightward after chronic dexamethasone exposure and normalize with metformin, providing quantitative EC₅₀ values in real time(3).
High-throughput formats are now feasible. Compera et al. integrated large-scale pneumatic valves to create a 96-unit micro-adipose array; alternating high/low glucose pulses every six hours generated time-resolved proteomic maps (> 1 800 proteins) without compromising viability, indicating that multi-parameter omics screens can be run at scale on adipose tissue on chip technology(9). Because microfluidic perfusion permits repeated sampling without disturbing tissue architecture, longitudinal dose–response profiles and wash-out kinetics can be obtained from a single donor construct, aligning with 3Rs principles and reducing variability.
Collectively, the evidence shows that perfused adipose tissue on chip platforms faithfully replicate chronic inflammation, hypertrophy, inter-organ lipid flux and pharmacological responses, offering human-relevant read-outs that outperform static 3D cultures and traditional animal models in predictive fidelity.
Outlook: Towards Multi-Organ, Patient-Specific Metabolic MPS
Adipose tissue-on-chip systems have advanced significantly as human-relevant models for studying metabolic disease. However, several critical challenges remain that continue to shape the direction of future research.
One priority is the integration of adipose tissue within multi-organ circuits. Metabolic diseases such as obesity and NAFLD involve dynamic crosstalk between adipose tissue, liver, muscle, and endocrine organs. Adipose modules connected to hepatic or cancer chips have already revealed inter-tissue effects that static cultures cannot replicate. Fluidic coupling with controlled directionality will be essential for capturing systemic feedback loops and evaluating therapeutic interventions in a physiologically relevant context.
A second focus is the refinement of materials and sensing strategies. Transitioning from animal-derived matrices to defined, tunable hydrogels is key to improving reproducibility and regulatory compliance. Embedding oxygen, pH, and metabolite sensors into microfluidic chips allows continuous, non-invasive monitoring of metabolic function. These technologies provide real-time insights into adipocyte viability, lipolytic activity, and inflammatory shifts, parameters that are otherwise difficult to measure in vitro.
Lastly, efforts are moving toward scalable, genotype-specific platforms for personalized drug discovery. Microfluidic arrays seeded with hiPSC-derived adipocytes enable comparative studies across patient backgrounds, supporting precision applications in metabolic screening. High-content omics, longitudinal readouts, and minimal cell requirements position these systems as ideal tools for high-throughput therapeutic evaluation.
Together, these developments signal a transition from isolated adipose constructs to modular, multi-organ MPS designed for predictive, human-based modeling. As standardization improves and integration advances, adipose-on-chip platforms are poised to play a central role in the future of NAMs.
Resources
- Contessi Negrini N, Pellegrinelli V, Salem V, Celiz A, Vidal-Puig A. Breaking barriers in obesity research: 3D models of dysfunctional adipose tissue. Trends Biotechnol. mai 2025;43(5):1079‑93.
- Liu Y, Kongsuphol P, Chiam SY, Zhang QX, Gourikutty SBN, Saha S, et al. Adipose-on-a-chip: a dynamic microphysiological in vitro model of the human adipose for immune-metabolic analysis in type II diabetes. Lab Chip. 2019;19(2):241‑53.
- Rogal J, Binder C, Kromidas E, Roosz J, Probst C, Schneider S, et al. WAT-on-a-chip integrating human mature white adipocytes for mechanistic research and pharmaceutical applications. Sci Rep. 20 avr 2020;10(1):6666.
- Kongsuphol P, Gupta S, Liu Y, Bhuvanendran Nair Gourikutty S, Biswas SK, Ramadan Q. In vitro micro-physiological model of the inflamed human adipose tissue for immune-metabolic analysis in type II diabetes. Sci Rep. 20 mars 2019;9(1):4887.
- Huff LK, Amurgis CM, Kokai LE, Abbott RD. Optimization and validation of a fat-on-a-chip model for non-invasive therapeutic drug discovery. Front Bioeng Biotechnol. 25 juin 2024;12:1404327.
- Slaughter VL, Rumsey JW, Boone R, Malik D, Cai Y, Sriram NN, et al. Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical therapeutic efficacy evaluation. Sci Rep. 23 juin 2021;11(1):13159.
- Baganha F, Schipper R, Hagberg CE. Towards better models for studying human adipocytes in vitro. Adipocyte. 31 déc 2022;11(1):413‑9.
- Leung CM, Ong LJY, Kim S, Toh YC. A physiological adipose-on-chip disease model to mimic adipocyte hypertrophy and inflammation in obesity. Organs—Chip. déc 2022;4:100021.
- Compera N, Atwell S, Wirth J, Von Törne C, Hauck SM, Meier M. Adipose microtissue-on-chip: a 3D cell culture platform for differentiation, stimulation, and proteomic analysis of human adipocytes. Lab Chip. 2022;22(17):3172‑86.
FAQ
Three-dimensional adipose organoids in a static state face two main constraints. First, diffusion is restricted. Oxygen and nutrient gradients develop, which leads to the formation of lipid-poor, multilocular adipocytes. The maintenance of unilocular white-fat morphology and stable adiponectin secretion is seldom achieved beyond two to three weeks. Second, mechanical and fluid cues are absent. Interstitial flow is not present. Adipocytes are not subjected to the shear, convective transport, or continual endocrine clearance that define depots within the body. These factors reduce the physiological relevance of static models.
Both shortcomings of static models are addressed by microfluidic "fat-on-chip" systems. Adipocytes are housed in perfused chambers. These chambers are hydraulically isolated from damaging shear. A continuous supply of medium is provided. A study by Liu and co-workers showed that a laminar flow of 8 nL s⁻¹ sustained the culture of differentiating human pre-adipocytes for weeks. Lipid droplets expanded under this constant nutrient exchange and waste removal. A different "WAT-on-a-chip" design by Rogal accommodated mature primary adipocytes. Shielding side channels were included so the buoyant cells stayed viable. A vascular-like conduit permitted online monitoring of fatty-acid uptake and isoproterenol-induced lipolysis.
Immunometabolic investigation is made possible by fluid flow. In a model of inflamed adipose tissue created by Kongsuphol, peripheral-blood mononuclear cells (PBMCs) were co-cultured with adipocytes on the chip. Time-resolved cytokine waves (IL-6, TNF-α) were produced. A drop in insulin-stimulated glucose uptake was also seen at the same time. These are observations not easily captured in static transwells. In a separate study by Huff et al., vascular integration was improved. Endothelial cells were embedded across a 3 µm porous membrane. Molecular diffusion was managed by the endothelial barrier, and over 90 per cent cell viability was maintained. Non-invasive sampling of triglycerides was also permitted.
Yes, perfused adipose chips can be networked with other organ models. An adipose-liver circuit was described by Slaughter. It was seen that effluent rich in free fatty acids, coming from hypertrophic fat, worsened hepatocyte steatosis. Activity of CYP3A4 was also altered. These are inter-organ responses not seen in isolated cultures. These platforms are considered microphysiological systems. They are accepted by regulators as new approach methodologies (NAMs) for metabolic disease research. The transition to perfused chips yields more mature lipid architecture. It also provides access to immune and inter-organ crosstalk.
The cellular composition is a main design principle. Pre-adipocyte lines were used in early devices, but lipid accumulation remained modest. Later studies used patient-derived stromal-vascular fractions (SVF) or purified mature adipocytes. It was shown by Rogal et al. that buoyant primary adipocytes can be stabilized within shallow micro-cavities. Unilocular morphology and leptin secretion were preserved for three weeks under flow. Similar stability was reached by Huff et al. with SVF-derived adipocytes differentiated in hydrogels. For brown-fat studies, ADSC or hiPSC progenitors are seeded on-chip. They can be thermogenically prompted to form UCP1\-positive constructs.
The chip’s vascular architecture is another design principle. Two main strategies are used. One is the parallel-channel design. In this setup, an endothelialized micro-channel is placed next to the adipose chamber. Nutrients and cytokines diffuse across a porous membrane. The adipocytes are kept shielded from damaging shear, specified as less than 0.05 dyn cm⁻². The second strategy is the self-assembled niche. Endothelial cells are embedded within the matrix itself. Capillary-like meshes are formed by spontaneous sprouting, which supports hypertrophy and improves insulin responsiveness. With both geometries, continuous sampling of effluent for adipokines, glycerol, and non-esterified fatty acids is allowed.
Depot identity is modulated by matrix selection. The formation of large unilocular droplets is encouraged by soft (≈2 kPa) collagen- or HA-based hydrogels. This morphology is typical of white adipose tissue. Stiffer GelMA or PEG-RGD formulations are different. They bias differentiation toward beige/brown phenotypes. This process happens via YAP-mediated mechanosignalling. Compositional fidelity can be found in decellularized adipose ECM, but donor variability is also introduced. Photo-tunable synthetic matrices are also available. Stiffness ramps during culture are permitted by these materials, which can be used to represent progressive fibrosis.
Inflammatory insulin resistance is a main application for these models. In the system described by Kongsuphol et al., peripheral-blood mononuclear cells were perfused through adipocytes derived from SVF. This action caused ten-fold surges in TNF-α and IL-6. Insulin-stimulated glucose uptake was also reduced by 60 per cent. This observation closely represents dysfunction linked to early obesity. In another study, cyclic high-glucose pulses were layered onto a chip by Liu et al.. Sustained serine phosphorylation of IRS-1 was produced by the combination of immune flow and glucotoxic stress. This response was not seen in static spheroids.
The translational usefulness of these models is shown by drug-screening applications. On the inflamed chip from Kongsuphol, the dual PPARα/δ agonist elafibranor was tested. IL-6 secretion was normalised. Over 80 per cent of insulin sensitivity at 10 µM was also restored. In static transwells, identical dosing showed only marginal benefit. Non-invasive mitochondrial stress testing was performed on Huff’s platform. Palmitate-induced reductions in spare-respiratory capacity were reversed by rosiglitazone within 48 hours. It was also shown by Rogal that isoproterenol-stimulated lipolysis curves are shifted rightward after chronic dexamethasone exposure. These curves are normalized with metformin, which produces quantitative EC₅₀ values in real time.
Some challenges remain that shape future research directions. One priority is placing adipose tissue within circuits containing other organs, such as the liver, muscle, and endocrine models. Fluidic coupling with controlled directionality will be needed for representing feedback loops. A second focus is on improving materials and sensing. The use of defined, tunable hydrogels instead of animal-derived matrices is needed for better reproducibility. Oxygen, pH, and metabolite sensors can be embedded into the chips. Continuous, non-invasive monitoring of metabolic function is permitted by these sensors. Efforts are also directed at genotype-specific platforms for drug screening. Microfluidic arrays seeded with hiPSC-derived adipocytes are used for comparative studies across patient backgrounds.