Introduction
Obesity and obesity-related co-morbidities are on the rise globally, necessitating the development of novel treatments. White adipose tissue (WAT) is a key regulator of whole-body metabolism and energy balance, as well as a key role in the development of insulin resistance and type 2 diabetes.
This study describes a new technique to isolate and culture mature human adipocytes. White adipose tissue (WAT) dysregulation plays a central role in the development of insulin resistance and type 2 diabetes (T2D). To develop new treatments for T2D, more physiologically relevant in vitro adipocyte models are required.
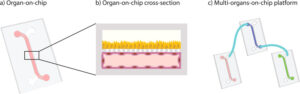
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The authors state that “Research on white adipose tissue (WAT), which constitutes one-fifth to one-half of the total body mass of a human’s body, has gained more and more interest and attention in the era of “diabesity”.
In vitro research on mature human WAT is hampered by many challenges and, hence, a majority of WAT-related research is conducted using animal models as well as clinical observations and genome-wide association studies (GWAS), both featuring limitations in terms of translatability and potential for experimental interventions, respectively.
Here, we describe methods to isolate primary mature human adipocytes from biopsies and to fabricate tailored organ-on-chip platforms for the long-term culture of WAT constructs.”
References
Rogal J, Roosz J, Loskill P. Isolation, Integration, and Culture of Human Mature Adipocytes Leveraging Organ-on-Chip Technology. Methods Mol Biol. 2022;2373:297-313. doi: 10.1007/978-1-0716-1693-2_18. PMID: 34520020.
FAQ
The global rise of obesity and its associated health conditions has created a need for new treatments. White adipose tissue, or WAT, is an important controller of the entire body’s metabolism. It also manages energy balance. Dysregulation of WAT is a main factor in the development of insulin resistance. This condition is linked to type 2 diabetes. Because of its connection to these widespread metabolic disorders, research into WAT has become more frequent. A better understanding of this tissue is needed to develop effective therapies. This tissue constitutes a large portion of a human’s total body mass, from one-fifth to one-half.
Research conducted in vitro on mature human white adipose tissue is impeded by many difficulties. These problems have led most WAT-related research to be conducted using other methods. Non-human models are frequently used. Clinical observations and genome-wide association studies, or GWAS, are also common approaches. These alternatives, however, are known to have limitations. Findings from non-human models often have problems with translatability. This means the results may not apply to people. Clinical studies and GWAS have restrictions on the types of experimental interventions that can be performed. Therefore, new in vitro models are required.
This study describes a new procedure to isolate and maintain mature adipocytes from humans. To develop new treatments for conditions like type 2 diabetes, more physiologically appropriate in vitro adipocyte models are needed. This paper details methods to help meet that need. First, a technique is described for isolating primary mature human adipocytes. These cells are taken from biopsies. Second, the study describes how to fabricate tailored organ-on-chip platforms. These platforms are specifically designed for the long-term maintenance of WAT constructs. This allows the tissue to be studied in a controlled laboratory setting over an extended period.
More physiologically relevant in vitro adipocyte models are required to develop new treatments. This is particularly true for conditions like type 2 diabetes, where white adipose tissue dysregulation is a main factor. Current research methods, such as non-human models and clinical observations, have specific drawbacks. These methods present issues with translatability or have limits on experimental interventions. Better laboratory models are needed to study the tissue directly. The methods described in this paper, which use organ-on-chip platforms, are intended to provide a more representative model. This could help researchers study human WAT in a more controlled and applicable way.