Introduction
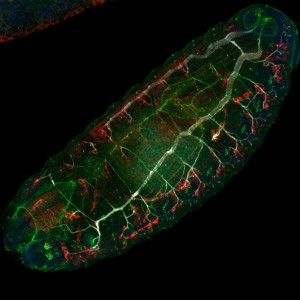
Drosophila embryo development or embryogenesis is a fast process during the life cycle of the d. melanogaster, completed in 24h after egg fertilization, and followed by three larval stages. Key steps in the body plan formation of the future adult fly occur during this stage.
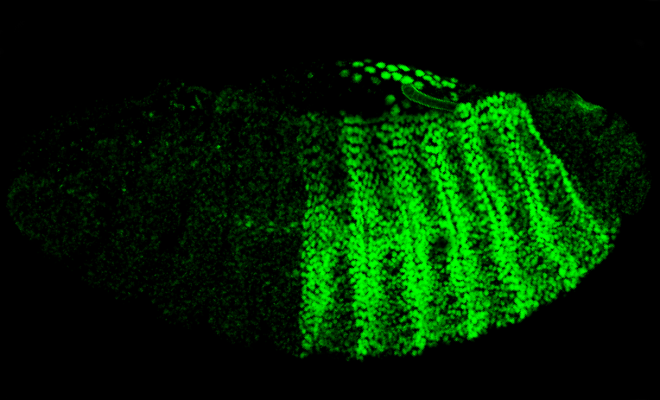
Drosophila embryo development
The deciphering of the developmental steps leading to the formation of the different tissue structures from the syncytial embryo has been possible through the multiple genetic tools available for the fruit fly model organism. Live imaging of the fly embryo has been as well an invaluable resource for the understanding of these processes.
Ultra fast temperature shift device for in vitro experiments under microscopy
Live imaging of D. melanogaster embryo
Confocal microscopy allows for the imaging of fluorescently labelled tissues during the different stages of drosophila development. Live imaging of d. melanogaster embryos requires non-invasive mounting procedures. Size (wt are 511±21 μm in length), thickness (wt are 184±5 μm in width), and optical properties are elements to take into consideration to properly mount the embryos for imaging [3]. Any mounting method requires a prior step of dechorionation of embryos, followed by placement of the embryos in the desired orientation depending on the experiment (dorsolateral, ventrolateral, posterior or anterior side).
The choice of the imaging mounting method depends on several factors, according to the biological process being studied: duration of the timelapse, is gas permeability needed or not, is squeezing of the embryo suitable or not. For instance, for morphogenesis studies, methods which require embryo squeezing would not be the most suited [8]. Dehydration, hypoxia and tissue deformation are important factors to be taken into consideration.
Many of the mounting methods require the use of halocarbon oil. Halocarbon oil prevents embryo dehydration and is oxygen permeable. Additionally, due to its physical properties, it helps to prevent motion of the embryos during time lapse imaging.
Some commonly used mounting methods for live imaging of fly embryo are:
1. Mounting between a glass slide and a glass coverslip: embryos in halocabon oil are placed in a sandwich between a glass slide and coverslip. Different methods allow to create imaging chambers by using spacers of the desired thickness (eg: glass coverslips, double sided tape).
2. Glass bottom Petri dish: embryos are mounted on the dish with halocarbon oil [4]. The main advantage of this method is the possibility of adding big volumes covering the embryo, which prevents evaporation and allows for longer imaging. Glass bottom petri dishes with different types of coating are commercially available.
3. Chambered coverslips: The main advantage of these chambers is the possibility of imaging multiple chambers on a single glass coverslip (high resolution imaging compatible), allowing for multiplexed experiments. As in the case of Petri dishes, these chambers allow the addition of big volumes, thus preventing evaporation. Multichambered coverglasses are commercially available.
4. Permeable membranes and stainless-steel imaging slides: embryos are placed between a membrane surface and the glass coverslip. The main advantage of this mounting method is the gas permeability provided by the membrane (made of materials like Teflon or lummox), allowing for long imaging with no risk of hypoxia for the tissues [6,7]. Petri dishes with a membrane-bottom and compatible with high resolution imaging are commercially available. Alternatively, stainless-steel imaging slides or Petri dishes with a membrane bottom can be prepared in the laboratory [5, 7].
5. Hanging drop protocol: it consists on a humid chamber where the embryo is placed on a halocarbon oil drop with no squeezing [8]. It doesn’t require compression of the embryos. Compatible with upright microscope setups.
The CherryTemp temperature controller is compatible with mounting methods 1,2 and 5. and we provide alternative solutions for compatibility with method 4 (gas permeability). Learn more about CherryTemp.
References
- [1] Hales, KG. Genetics (2015) https://www.ncbi.nlm.nih.gov/pubmed/26564900
- [2] https://en.wikipedia.org/wiki/Eric_F._Wieschaus
- [3] Mavrakis M. et al. Curr Protoc Cell Biol. (2008). https://www.ncbi.nlm.nih.gov/pubmed/18551421
- [4] Jankovics F. ¬Dev Cell. (2006) https://www.ncbi.nlm.nih.gov/pubmed/16908221
- [5] Kiehart DP et al. Methods Cell Biol. (1994) https://www.ncbi.nlm.nih.gov/pubmed/7707969
- [6] Kiehart DP et al., J Cell Biol. (2000) https://www.ncbi.nlm.nih.gov/pubmed/10769037
- [7] Reed BH et al. Curr Biol. (2004) https://www.ncbi.nlm.nih.gov/pubmed/15028211
- [8] Reed BH et al. J Vis Exp. (2009) https://www.ncbi.nlm.nih.gov/pubmed/19287353
FAQ
Drosophila embryo development, or embryogenesis, is a quick process that is completed in 24 hours after egg fertilisation. It is followed by three larval stages. Early development happens in a syncytium. In this state, nuclear divisions and very rapid DNA replication occur, but cell divisions do not. After 6000 nuclei have migrated to the periphery of the syncytium, cellularisation begins. The syncytial blastoderm is then generated. Next, in a process called gastrulation, cell shapes are changed and cells migrate. The germ layers of the future body plan (mesoderm, endoderm, and ectoderm) are established by this action. The process of cell fate determination is directed by underlying patterned gene expression. This expression pattern is established at the blastoderm stage, prior to gastrulation.
Live imaging of D. melanogaster embryos requires non-invasive mounting procedures. Several points must be considered, based on the biological process being studied. These points include the duration of the timelapse and whether gas permeability is needed. It is also necessary to determine if squeezing of the embryo is suitable. For instance, methods that require embryo squeezing would not be the most appropriate for morphogenesis studies. Dehydration, hypoxia, and tissue deformation are other important issues that must be taken into consideration. Before any mounting method is used, a prior step of dechorionation of the embryos is required. This is followed by placing the embryos in the desired orientation, such as dorsolateral or ventrolateral, depending on the specific experiment.
The use of halocarbon oil is required by many mounting methods for Drosophila embryos. This substance serves several functions. Dehydration of the embryo is prevented by the halocarbon oil during the imaging process. The oil is also permeable to oxygen. Hypoxia in the tissues can be avoided during long imaging sessions due to this property. Additionally, the physical properties of the oil are useful for time-lapse imaging. Motion of the embryos is prevented by the oil. This ensures the embryo remains in place while images are acquired. For example, embryos in halocarbon oil are placed in a sandwich between a glass slide and coverslip. They are also used for mounting on a glass bottom Petri dish. In the hanging drop protocol, the embryo is also placed in a halocarbon oil drop.
Several mounting methods are commonly used for the live imaging of fly embryos. One method involves mounting between a glass slide and a glass coverslip. For this technique, embryos in halocarbon oil are placed in a sandwich configuration. Spacers of a desired thickness, such as double-sided tape, can be used to create imaging chambers. In another method, a glass bottom Petri dish is used, and embryos are mounted on it with halocarbon oil. The addition of large volumes is possible with this method, which prevents evaporation and allows for longer imaging. Chambered coverslips provide similar advantages, allowing multiplexed experiments on a single glass coverslip. Permeable membranes made of materials like Teflon are also used to provide gas permeability for long imaging sessions without hypoxia risk.