Introduction
The fruit fly Drosophila melanogaster (D. melanogaster) has been widely used as a model organism in biological research, particularly in genetic and developmental studies, since the early 20th century. In laboratories, the term drosophila is often used to refer to the species D. melanogaster. Although thousands of species exist in the genus Drosophila, D. melanogaster is the most widely used in research.

D. melanogaster as a model organism
D. melanogaster is a widely used model organism because of its easy manipulation and growth in laboratory conditions in all the stages of its life cycle, from egg to adult. The developmental cycle of drosophila depends on temperature, being of 8.5 to 9 days at 25°C and being slowed down at higher and lower temperatures because of suboptimal growth [1].
Fruit flies are yellow/brown colored with red eyes. They show sexual dimorphism which allows for an easy identification of males and females in the laboratory. Some of the traits of this sexual dimorphism are: females are bigger than males, differences in color, differences in the black patterning in the abdomen, claspers around male genital structures and sexcomb bristles in the male legs [1,12].
The use of the fruit fly as model organism has been particularly important in genetics, developmental biology, and behavioral studies.
Ultra fast temperature shift device for in vitro experiments under microscopy
Genetic studies in the fruit fly
The availability of multiple genetic tools made drosophila an ideal model system to decipher gene function and molecular pathways. Some examples of these tools are: the easy generation, through genetic crosses and offspring selection, of stable genetic stocks, the powerful genetic screens with mutagenesis or transposone insertion for gene disruption, the use of genetic transformation, the availability of tools allowing for conditional gene expression (GAL4/UAS system), the use of RNAi for conditional knock down and the more recent genome editing CRISPR/Cas9 technology [1].
Drosophila has a small genome of 180Mb with around 13600 genes. The full genome has been sequenced [2]. A global picture about gene expression, chromatin regulation, DNA replication, splicing (around half of the drosophila genes are regulated through alternative splicing), through the different developmental stages has contributed to the understanding of drosophila’s developmental regulation [3].
The genome of D. melanogaster is distributed in only 4 chromosomes (3 autosomes and one pair of sex chromosomes). One of the autosome pairs, known as the dot chromosome, is significantly smaller than the others [4]. The study of the sex chromosomes in drosophila has contributed to the understanding of sex determination and dosage compensation. In flies, the dose of X chromosomes in the chromosome complement is the responsible of male determination, and not the presence of the Y chromosome as it occurs in humans. The double X chromosome sensing in drosophila is made at the cellular level, and it triggers a transcriptional cascade leading to sex determination [5].
Developmental biology and behavioral studies using melanogaster
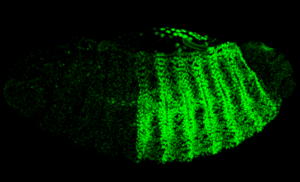
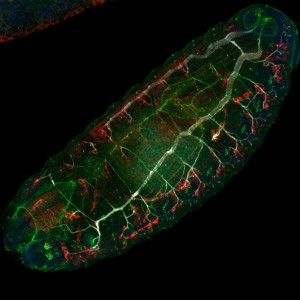
The use of melanogaster for developmental biology started with the seminal discoveries of the Hox genes as responsible of pattern formation and body plan [6]. Since then, the whole process of transformation of an embryo to a complex animal has been widely studied: from rapid divisions in the syncytial embryo, tissue development during embryogenesis, larval growth and pupal stages, till the formation of adult structures from the imaginal disks. Confocal microscopy has been a key technology to allow the imaging of fluorescently labelled tissues during the different stages of drosophila development.
Research on the adult D. melanogaster has been as well of great importance. Adult fly lifespan is around 30 days (variable according to temperature). Multiple studies on different aspects of the animal physiology have been carried out, often linked to neurobiology: behavioral studies on reproductive court ceremonies and sexual behavior [10], studies on sleep patterns and circadian clocks [7, 9] and ageing [8, 11]. Additionally, since molecular pathways are highly conserved through eukaryotes and many disease-causing genes in humans are conserved in the fly, drosophila has been used as an animal model for research on human disease, including cancer, neurological disorders, and rare diseases.
References
- [1] Dillon ME, Wang G, Garrity PA, Huey RB. Review: Thermal preference in Drosophila. J Therm Biol [Internet]. (2009) 34(3):109–19. Available here
- [2] Brown P. A review of techniques used in the preparation, curation and conservation of microscope slides at the Natural History Museum, London. Biol Curator [Internet]. (1997) Available here
- [3] Champion De Crespigny FE, WEDELL N. The impact of anaesthetic technique on survival and fertility in Drosophila. Physiol Entomol [Internet]. (2008) 33(4):310–5.
- [4] Pavan C, da Cunha AB, Morsoletto C. Virus-Chromosome Relationships In Cells Of Rhynchosciara (Diptera, Sciaridae). Caryologia [Internet]. (1971) 24(3):371–89. Available here
- [5] Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, et al. The genome sequence of Drosophila melanogaster. Science [Internet]. (2000) 287(5461):2185–95. Available here
- [6] Zatsepina OG, Velikodvorskaia V V, Molodtsov VB, Garbuz D, Lerman DN, Bettencourt BR, et al. A Drosophila melanogaster strain from sub-equatorial Africa has exceptional thermotolerance but decreased Hsp70 expression. J Exp Biol [Internet]. (2001) 204(Pt 11):1869–81. Available here
- [7] Chakir M, Chafik A, Moreteau B, Gibert P, David JR. Male sterility thermal thresholds in Drosophila: D. simulans appears more cold-adapted than its sibling D. melanogaster. Genetica [Internet]. (2002) 114(2):195–205. Available here
- [8] Schweisguth F. Dominant-negative mutation in the beta2 and beta6 proteasome subunit genes affect alternative cell fate decisions in the Drosophila sense organ lineage. Proc Natl Acad Sci U S A [Internet]. (1999) 96(20):11382–6. Available here
- [9] McEvey, Shane (2017). High resolution diagnostic images of Drosophila suzukii (Diptera: Drosophilidae). figshare. Figure. https://doi.org/10.6084/m9.figshare.4644793.v1
FAQ
D. melanogaster is often used as a model organism. This is because it is easy to manipulate and grow in laboratory conditions. These conditions apply to all stages of its life cycle, from egg to adult. The fruit fly has a developmental cycle that is dependent on temperature. At 25°C, this cycle takes 8.5 to 9 days. Growth is slowed at higher and lower temperatures. The flies also show sexual dimorphism, which allows for easy identification of males and females. Females are larger than males, and there are differences in abdominal patterning and colour. Males also possess claspers and sexcomb bristles. The model is used in genetic, developmental, and behavioural studies.
The fruit fly is a suitable model for deciphering gene function. This is due to the availability of multiple genetic tools. It is easy to generate stable genetic stocks through crosses and selection. Effective genetic screens using mutagenesis or transposon insertion for gene disruption can be performed. Other available tools include genetic transformation, RNAi for conditional knock-down, and the CRISPR/Cas9 genome editing technology. The GAL4/UAS system is also available, which allows for conditional gene expression. Drosophila has a small genome of 180Mb with about 13600 genes. This genome has been fully sequenced. A global picture of gene expression, DNA replication, and chromatin regulation throughout development has been established.
The genome of D. melanogaster is distributed in four chromosomes. These consist of three autosomes and one pair of sex chromosomes. The study of these sex chromosomes has contributed to the understanding of sex determination. In humans, the presence of the Y chromosome determines the male sex. This is not the case for flies. Instead, the dose of X chromosomes in the chromosome complement is responsible for male determination. The sensing of the double X chromosome is done at the cellular level. This sensing action then initiates a transcriptional cascade, which leads to the final sex determination.
The use of melanogaster for developmental biology began with foundational discoveries of the Hox genes. These genes are responsible for pattern formation and the body plan. The whole process of transformation from an embryo to a complex animal is widely studied. This includes rapid divisions in the syncytial embryo, tissue development, larval growth, and the formation of adult structures from imaginal disks. Confocal microscopy is an important technology, as it allows for the imaging of fluorescently labelled tissues during these stages. Research on the adult fly is also conducted. The adult lifespan is around 30 days, though this varies with temperature. Many studies are linked to neurobiology. These include studies on sexual behaviour, sleep patterns, circadian clocks, and ageing.