Breast cancer has long been one of the most prevalent and challenging cancers worldwide, demanding constant advances in treatment and research. From radical surgeries like the Halsted mastectomy to the emergence of targeted drugs, every decade has brought new insights into how to better match therapy to each patient’s unique disease. Today, the breast cancer organoid platforms represent the next step in this evolution; an in vitro model that faithfully recreates a patient’s tumor outside the body. This article traces the breast cancer treatment history from the early days of breast cancer care to the promise of organoids in driving true precision oncology.
Breast Cancer and Phenotype Diversity
Breast cancer remains the most common malignancy among women worldwide, with approximately 2.3 million new cases diagnosed each year and nearly 15% of all female cancer deaths attributed to it(1–3). While survival rates have improved significantly thanks to early detection and better therapies, breast cancer continues to pose unique clinical challenges because it is not a single disease, but rather a collection of highly diverse subtypes with distinct biological behaviors.
This diversity is rooted in the molecular landscape of breast tumors. Decades ago, treatment decisions were primarily based on tumor size and stage. However, a deeper understanding of breast cancer biology has revealed that its molecular characteristics (such as hormone receptor status, HER2 amplification, and genomic instability) play an equally critical role in determining prognosis and response to therapy(1–3).
One of the key advances in this area was the intrinsic molecular classification proposed by Perou et al in 2000, which distinguished breast cancers into at least four major subtypes: luminal A, luminal B, HER2-enriched, and basal-like (often overlapping with triple-negative breast cancer, or TNBC)(4). Clinically, this has been further refined into surrogate subtypes, helping oncologists match each tumor’s biology to the most effective therapies.
For example, hormone receptor-positive (HR+) tumors, which express estrogen receptor (ER) and/or progesterone receptor (PR), account for up to 65% of cases and often respond well to endocrine therapies like tamoxifen or aromatase inhibitors. Conversely, HER2-positive tumors, which make up about 20% of cases, tend to be more aggressive but can be effectively targeted with anti-HER2 drugs such as trastuzumab. Triple-negative breast cancer (defined by the lack of ER, PR, and HER2) represents around 15% of cases and is associated with poorer prognosis and higher rates of recurrence, partly because fewer targeted options exist for this group(1–3).
Adding to the complexity, breast cancer phenotypes can shift under the selective pressure of therapy or metastasis. Xiong et al. highlight how receptor status may change during disease progression or after neoadjuvant chemotherapy, requiring periodic reassessment to ensure treatment remains appropriately tailored(3). Moreover, intra-tumoral heterogeneity, the coexistence of multiple cell clones within a single tumor, means that resistance can emerge even when an initial response is promising.
This diversity in tumor biology underscores the importance of precision medicine in breast cancer care. By recognizing that a “one-size-fits-all” approach is inadequate, modern oncology increasingly focuses on matching each patient’s treatment plan to the unique molecular makeup of their disease. However, as we will see in the next section, the journey toward truly personalized therapy has evolved through decades of incremental breakthroughs, and it still faces significant limitations that call for new experimental models, including patient-derived breast cancer organoid technology.
Learn more about our ready to use breast cancer organoid models.
A Short History of Breast Cancer Treatment and the Rise of Precision Medicine
The story of breast cancer treatment has always reflected the broader evolution of oncology, a gradual move from one-size-fits-all interventions to increasingly tailored strategies that respect the disease’s underlying biology.
In the early 20th century, the mainstay of breast cancer care was radical surgery. The Halsted mastectomy, which removed not only the breast but also underlying chest muscle and regional lymph nodes, was performed in the hope of stopping cancer from spreading. For decades, this extensive approach was widely accepted, but studies later showed that it did not significantly improve survival for many patients compared to less aggressive surgery. Clinical trials led by pioneers such as Bernard Fisher helped demonstrate that breast-conserving surgery combined with radiotherapy could achieve outcomes equal to or better than radical mastectomy for early-stage cancer, but with fewer physical and psychological consequences for patients(6).
This shift from “maximum tolerated treatment” to “minimum effective treatment” laid the groundwork for a new era: the use of systemic therapies based on the unique features of each patient’s disease. In the late 1970s and early 1980s, tamoxifen (the first targeted hormone therapy) began to transform the management of hormone receptor-positive breast cancer. Its success made it clear that understanding the molecular profile of a tumor could have a real clinical impact. Soon after, combination chemotherapy regimens and targeted biologic agents followed, adding new tools to delay recurrence and improve survival.
A major turning point came with the discovery of the HER2 gene amplification in certain aggressive forms of breast cancer. This led to the development of trastuzumab, the first monoclonal antibody to target HER2-positive tumors. The introduction of HER2-targeted therapies demonstrated that survival could be dramatically improved for patients whose tumors carried this specific biomarker, highlighting the power of precision medicine in action(7).
Today, routine molecular profiling for hormone receptors, HER2 status, and increasingly for additional genomic alterations like BRCA and PIK3CA mutations, guides treatment recommendations for many patients. Multigene assays can also estimate recurrence risk and help avoid overtreatment when chemotherapy offers little benefit. However, the promise of “the right drug for the right patient at the right time” remains only partially fulfilled. Not all patients benefit equally from precision strategies because many genomic alterations do not yet have proven, effective drugs to target them.
For example, recent work by André et al. showed that patients with metastatic breast cancer who received targeted treatments matched to well-validated genomic alterations experienced significantly longer progression-free survival than those who did not. But importantly, this benefit was limited to patients whose tumors had alterations that had already been shown in trials to predict a response to treatment. Patients with lower-confidence genomic variants did not see the same improvement, highlighting the limits of current approaches and the need for more functional, patient-specific testing platforms(8).
As Liu et al.point out, tumor heterogeneity and acquired resistance remain major obstacles to long-term treatment success(9). Even with the best current tests, many patients do not have an actionable alteration, or their tumors adapt quickly to targeted drugs. As the search for more effective strategies continues, new experimental models are urgently needed to help bridge this gap, and patient-derived breast cancer organoid platforms are one of the most promising tools on the horizon.
Breast Cancer Organoid : A Promising New Platform for Precision Medicine
As breast cancer care continues to move toward individualized treatment, one of the greatest challenges remains finding the right therapy for the right patient at the right time, especially when existing molecular markers provide limited guidance. In recent years, patient-derived organoids have emerged as one of the most promising tools to help close this gap.
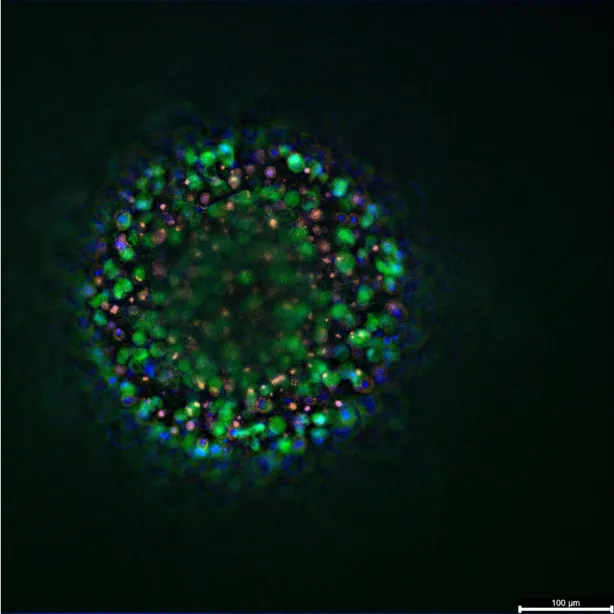
Patient-derived organoids are three-dimensional, miniaturized tissue cultures grown from a patient’s own cells. Unlike traditional cell lines, which often lose key features of the original tumor over time, organoids maintain the unique genetic, structural, and functional characteristics of the tissue they come from(10). This means they can more faithfully replicate the biological behavior of an individual patient’s tumor in the lab, offering a highly relevant model for testing how that tumor might respond to various treatments.
This technology builds on decades of advances in 3D cell culture and stem cell biology. Initial organoid models were first developed to recreate healthy tissue architecture, but in oncology, the focus quickly shifted to cultivating patient-derived tumor organoids (PDTOs) that can act as living “avatars” for drug testing and mechanistic research(11). Today, PDTOs have been successfully generated for many cancers (including breast cancer) with high success rates, and protocols are constantly improving to make them faster and more reproducible(12).
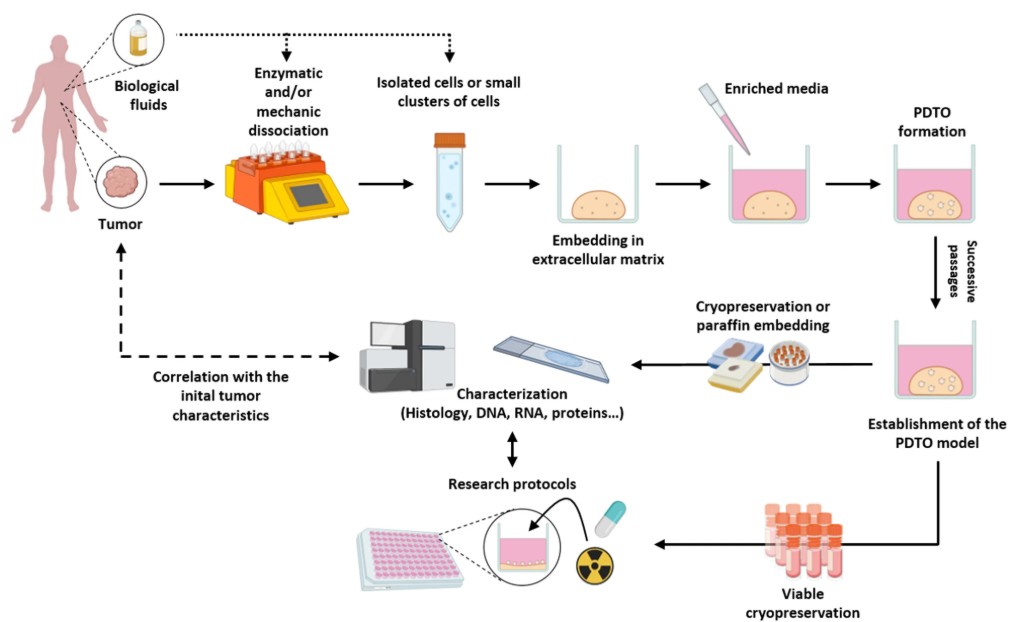
Breast cancer organoid systems have attracted growing interest because they can capture the remarkable heterogeneity of this disease. Researchers have demonstrated that organoids can be grown from different breast cancer subtypes, including hormone receptor-positive, HER2-positive, and triple-negative tumors. These models preserve the distinct molecular features that influence prognosis and treatment response(12). This fidelity makes organoids an invaluable tool for preclinical drug screening, helping researchers test how specific combinations of therapies may work or fail in an individual’s tumor before administering them in the clinic.
Beyond drug testing, organoids are opening new doors for understanding the fundamental biology of breast cancer. Studies are using them to investigate how tumor cells interact with the surrounding microenvironment, adapt under therapy, or acquire drug resistance over time. And as Verstegen et al. point out, organoids are increasingly being integrated with other cutting-edge technologies, such as CRISPR-based gene editing, multi-omics profiling, and organ-on-chip platforms(11). These advances aim to simulate the dynamic conditions of a living human body even more realistically, which is especially important for cancers as diverse and adaptive as breast cancer.
Of course, there are still challenges to overcome. Establishing organoids from patient tissue requires fresh, viable samples and standardized methods to ensure consistent results. Not every tumor fragment will grow successfully, and some organoids still lack certain components of the tumor microenvironment. But technological progress is addressing these hurdles, with new co-culture systems that include stromal and immune cells to better mimic how tumors behave in the body(10).
Looking ahead, the goal is to make breast cancer organoid platforms a routine part of personalized medicine. By combining organoid-based drug testing with genomic profiling, clinicians may soon be able to design treatment plans that reflect not just what mutations a tumor has, but how it actually responds to therapy in real time. In doing so, breast cancer organoids have the potential to transform the promise of precision oncology into a practical reality for more patients.
Resources
- Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Primer. 23 sept 2019;5(1):66.
- Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. The Lancet. mai 2021;397(10286):1750‑69.
- Xiong X, Zheng LW, Ding Y, Chen YF, Cai YW, Wang LP, et al. Breast cancer: pathogenesis and treatments. Signal Transduct Target Ther. 19 févr 2025;10(1):49.
- Perou CM, Sørlie T, Eisen MB, Van De Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 17 août 2000;406(6797):747‑52.
- Orrantia-Borunda E, Anchondo-Nuñez P, Acuña-Aguilar LE, Gómez-Valles FO, Ramírez-Valdespino CA. Subtypes of Breast Cancer. In: Mayrovitz HN. editor. Breast Cancer. Brisbane (AU): Exon Publications. Online first 22 Jun 2022.
- Anderson S. A Short History of Bernard Fisher’s Contributions to Randomized Clinical Trials. Clin Trials. avr 2022;19(2):127‑36.
- Papalexis P, Georgakopoulou V, Drossos P, Thymara E, Nonni A, Lazaris A, et al. Precision medicine in breast cancer (Review). Mol Clin Oncol. 20 août 2024;21(5):78.
- Andre F, Filleron T, Kamal M, Mosele F, Arnedos M, Dalenc F, et al. Genomics to select treatment for patients with metastatic breast cancer. Nature. 13 oct 2022;610(7931):343‑8.
- Liu XY, Yu TJ, Shao ZM. Precision medicine for breast cancer: advances and challenges. Transl Breast Cancer Res. oct 2024;5:35‑35.
- Thorel L, Perréard M, Florent R, Divoux J, Coffy S, Vincent A, et al. Patient-derived tumor organoids: a new avenue for preclinical research and precision medicine in oncology. Exp Mol Med. 1 juill 2024;56(7):1531‑51.
- Verstegen MMA, Coppes RP, Beghin A, De Coppi P, Gerli MFM, De Graeff N, et al. Clinical applications of human organoids. Nat Med. févr 2025;31(2):409‑21.
- Huang S, Mei Z, Wan A, Zhao M, Qi X. Application and prospect of organoid technology in breast cancer. Front Immunol. 26 août 2024;15:1413858.
FAQ
Breast cancer is not a single illness. It is understood to be a collection of highly varied subtypes. These subtypes have distinct biological behaviours. Treatment decisions were once based only on tumour size and stage. It is now understood that molecular characteristics determine prognosis and therapy response. A classification system proposed in 2000 divides breast cancers into groups. These are luminal A, luminal B, HER2-enriched, and basal-like. These subtypes respond differently to treatment. Hormone receptor-positive tumours account for many cases and respond to endocrine therapies. HER2-positive tumours are different and are targeted with anti-HER2 drugs. This diversity means a "one-size-fits-all" approach is not adequate for treatment.
Tumours are classified by their molecular characteristics. Up to 65 per cent of cases are hormone receptor-positive (HR+). These tumours express the estrogen receptor or progesterone receptor. They often respond well to endocrine therapies, such as tamoxifen or aromatase inhibitors. About 20 per cent of cases are HER2-positive. These tumours tend to be more aggressive. They can be targeted with specific anti-HER2 drugs, like trastuzumab. Triple-negative breast cancer (TNBC) accounts for around 15 per cent of cases. This type is defined by the absence of ER, PR, and HER2. A poorer prognosis and higher rates of recurrence are associated with TNBC, partly because fewer targeted options exist for this group.
The diversity of tumour biology creates challenges for treatment. Phenotypes can shift. This happens under the selective pressure of therapy or during metastasis. This means receptor status may change during the progression of the disease or after chemotherapy. Reassessment is sometimes required. This is done to ensure the treatment remains tailored to the tumour’s current state. Another issue is intra-tumoural heterogeneity. This is the coexistence of multiple different cell clones within a single tumour. Because of this, resistance can emerge. This may happen even when an initial response to a drug is promising. These factors underscore the need for precision medicine approaches.
The story of breast cancer treatment shows a gradual move away from one-size-fits-all interventions. In the early 20th century, radical surgery was the main treatment. The Halsted mastectomy was performed. This procedure removed the breast, the underlying chest muscle, and regional lymph nodes. This extensive approach was widely accepted for decades. Later studies, however, showed that it did not improve survival for many patients compared to less aggressive surgery. Clinical trials demonstrated that breast-conserving surgery could be combined with radiotherapy. For early-stage cancer, outcomes were shown to be equal to or better than radical mastectomy, with fewer physical and psychological consequences.
A new era of systemic therapies began after the shift away from radical surgery. These therapies were based on the features of each patient’s disease. In the late 1970s and 1980s, tamoxifen began to be used. This was the first targeted hormone therapy. It changed the management of hormone receptor-positive breast cancer. The success of tamoxifen made it clear that the molecular profile of a tumour could have a clinical effect. A major development followed. This was the discovery of the HER2 gene amplification in certain aggressive tumours. This led to the creation of trastuzumab, the first monoclonal antibody to target HER2-positive tumours. This demonstrated that survival could be improved by targeting specific biomarkers.
The promise of matching the right drug to the right patient is only partially fulfilled. Molecular profiling for hormone receptors, HER2 status, and genomic alterations like BRCA and PIK3CA is now routine. These tests guide treatment recommendations. Not all patients, however, benefit equally from these strategies. Many genomic alterations do not yet have proven, effective drugs to target them. One study showed that patients with metastatic breast cancer received targeted treatments. Longer progression-free survival was experienced, but only if the treatments were matched to well-validated genomic alterations. Patients with lower-confidence genomic variants did not see the same improvement. Tumour heterogeneity and acquired resistance also remain major obstacles.
Patient-derived organoids are one of the tools being used to address gaps in precision medicine. They are defined as three-dimensional, miniaturized tissue cultures. They are grown from a patient’s own cells. A distinction is made between organoids and traditional cell lines. Cell lines often lose characteristics of the original tumour over time. Organoids, by contrast, are reported to maintain the unique genetic, structural, and functional characteristics of the tissue they come from. This allows them to replicate the biological behaviour of an individual patient’s tumour in a lab setting. They can act as living representations for drug testing and mechanistic research.
Breast cancer organoid systems are used because they can capture the heterogeneity of the disease. It has been shown that organoids can be grown from different breast cancer subtypes. These include hormone receptor-positive, HER2-positive, and triple-negative tumours. These models preserve the distinct molecular features that influence prognosis and treatment response. This fidelity makes organoids a useful tool for preclinical drug screening. Researchers can test how specific combinations of therapies may work or fail. This can be done in an individual’s tumour model before administration in the clinic. Organoids are also used to investigate how tumour cells interact with the surrounding microenvironment. Acquired drug resistance can be studied over time.
Challenges still need to be addressed. The establishment of organoids from patient tissue requires fresh, viable samples. Standardized methods are also needed. This is to ensure that results are consistent. It is not guaranteed that every tumour fragment will grow successfully. Some organoids also have limitations. They may still lack certain components of the tumour microenvironment. Technological progress is being made to address these hurdles. New co-culture systems are being developed. These systems include stromal and immune cells. The goal is to better mimic how tumours behave in the body.
Breast cancer is described as the most common malignancy among women worldwide. Approximately 2.3 million new cases are diagnosed each year. It is also responsible for nearly 15 per cent of all female cancer deaths. Survival rates have improved. This is attributed to early detection and better therapies. The disease continues to pose clinical challenges. This is because it is not a single disease. It is a collection of highly diverse subtypes. This diversity means that modern oncology must focus on matching a treatment plan to the unique molecular makeup of each patient’s disease.