Introduction
The state of the art for tissues-on-chips using skin cells had been lacking for a long time due to the absence of both an epithelial and a stromal component despite the biological importance of the stroma for the structure and function of human tissues.
The authors of this paper have presented a new inexpensive, easy-to-implement, versatile, and robust vinyl-based device that overcomes some of the drawbacks present in PDMS-based chips by using parallel flow and to mimic the function of a blood vessel as it is really important for a 3D model of tissues-on-chips using skin cells.
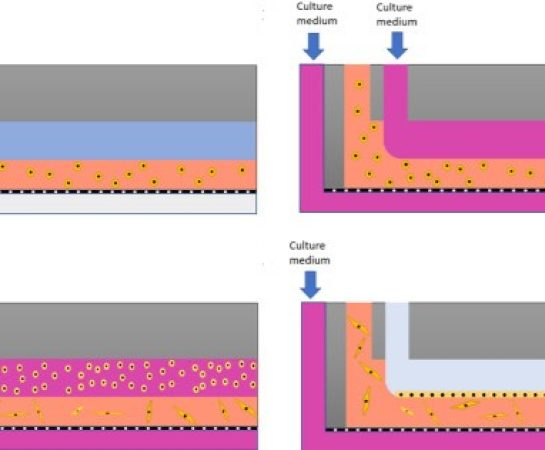
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract of a new microfluidic method enabling the generation of multi-layered tissues-on-chips using skin cells as a proof of concept..a
The authors state that “Microfluidic-based tissues-on-chips (TOCs) have thus far been restricted to modeling simple epithelia as a single cell layer, but likely due to technical difficulties, no TOCs have been reported to include both an epithelial and a stromal component despite the biological importance of the stroma for the structure and function of human tissues.
We present, for the first time, a novel approach to generate 3D multilayer tissue models in microfluidic platforms. As a proof of concept, we modeled skin, including a dermal and an epidermal compartment.
To accomplish this, we developed a parallel flow method enabling the deposition of bilayer tissue in the upper chamber, which was subsequently maintained under dynamic nutrient flow conditions through the lower chamber, mimicking the function of a blood vessel.
We also designed and built an inexpensive, easy-to-implement, versatile, and robust vinyl-based device that overcomes some of the drawbacks present in PDMS-based chips. Preliminary tests indicate that this biochip will allow the development and maintenance of multilayer tissues, which opens the possibility of better modeling of the complex cell-cell and cell-matrix interactions that exist in and between the epithelium and mesenchyme, allowing for better-grounded tissue modeling and drug screening.”
References
Valencia L, Canalejas-Tejero V, Clemente M, Fernaud I, Holgado M, Jorcano JL, Velasco D. A new microfluidic method enabling the generation of multi-layered tissues-on-chips using skin cells as a proof of concept. Sci Rep. 2021 Jun 23;11(1):13160. DOI: 10.1038/s41598-021-91875-z. PMID: 34162909; PMCID: PMC8222336.
FAQ
Previous microfluidic-based tissues-on-chips were limited. They were generally restricted to modelling simple epithelia, which consist of a single cell layer. A significant shortcoming, particularly for skin models, was the absence of both an epithelial and a stromal component. This omission was notable because the stroma has biological importance for the structure and function of human tissues. It is thought that technical difficulties prevented earlier models from including this component. The lack of a stromal element meant these models could not fully represent the biological complexity of actual tissue, which relies on interactions between these different cellular layers.
A new method for generating 3D multilayer tissue models in microfluidic devices is presented. As an initial model to demonstrate the method, skin was modelled with both a dermal and an epidermal compartment. A parallel flow method was developed to accomplish this. This technique enables the deposition of bilayer tissue within an upper chamber of the device. The tissue is subsequently maintained by circulating nutrient flow conditions. This nutrient flow passes through a lower chamber. This design is intended to replicate the function of a blood vessel, which is important for 3D tissue models.
A new vinyl-based device has been designed and built. This device is described as inexpensive and easy to implement. It is also considered versatile. This new design overcomes some of the drawbacks that are present in chips made from PDMS. It is a durable system. The chip was specifically designed to be used with a parallel flow method. This method is what allows for the creation of the bilayer tissue in one chamber, while a lower chamber provides nutrient access. The material and design choices were made to create a more accessible and reliable platform for tissue modelling.
Initial tests suggest that this biochip will permit the development and maintenance of multilayer tissues. This capability opens new possibilities for modelling complex biological interactions. The system may allow for better modelling of the cell-cell and cell-matrix interactions that occur within and between the epithelium and mesenchyme. Having both epithelial and stromal components is important for the structure and function of human tissues. By including these, more representative tissue modelling can be achieved. This, in turn, allows for better-grounded drug screening applications. The method provides a new tool for studying these intricate tissue systems.