
Obesity and its related metabolic diseases, such as type 2 diabetes, represent a growing global health crisis, yet progress in treatment has been hampered by a reliance on models that fail to faithfully recapitulate human adipose tissue biology. Over the past few years, major advances have been made through the adoption of human-derived 3D adipose tissue models, including organoids, spheroids, and organ-on-chip systems. These technologies capture complex cellular crosstalk, metabolic dysfunction, and therapeutic responses with a fidelity that surpasses traditional 2D cultures or animal models, while aligning with the 3Rs principles of reducing animal use.
Researchers are now integrating these advanced in vitro models with clinical data and computational approaches, creating a more comprehensive and predictive research framework. This strategy is not only being adopted in academic laboratories but is also strongly encouraged at the regulatory and industrial level. Agencies like the FDA and NIH have explicitly promoted such New Approach Methodologies (NAMs) as part of their roadmap to reduce reliance on animal testing, highlighting organoids and organ-on-chip systems as validated tools to accelerate translation and improve preclinical drug evaluation.
In this article, we highlight 10 key studies on adipose tissue research published in the last five years. Each demonstrates how innovative 3D adipose tissue models are driving research forward and paving the way toward more precise and patient-centered therapies for metabolic diseases.
Learn more about our ready to use Adipose organoid plate.
Fighting Cancer by Starving It with Fat
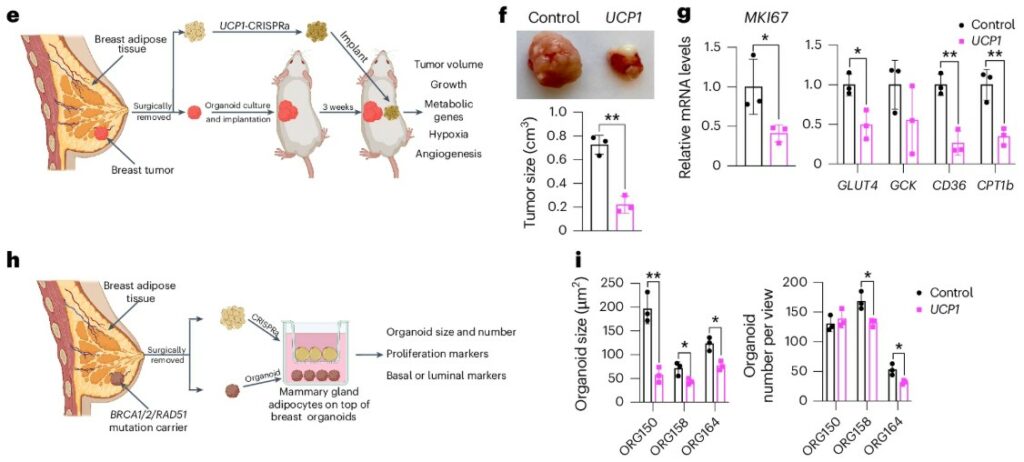
Cancer cells are voracious eaters. To fuel their rapid growth, they consume enormous amounts of nutrients like glucose and fatty acids from their immediate environment. While scientists have developed drugs to block these metabolic pathways, a more audacious idea has recently emerged: what if we could starve tumors by creating a competitor that is even greedier for nutrients? Studies showed that activating the body’s natural energy-burning brown fat could slow cancer progression, but the methods, like prolonged cold exposure, were not practical for patients. This created a pressing need for new approach methodologies to build a targeted, implantable “nutrient sink” that could wage metabolic war on tumors.
This study introduces a brilliant solution using a living therapeutic. The researchers used CRISPR to engineer standard white fat cells to over-express UCP1, a gene that transforms them into highly metabolic, energy-burning “beige” adipocytes. Critically, they grew these cells into a stable, 3D adipose tissue organoid. This organoid structure is far more robust and tissue-like than individual cells, making it ideal for transplantation. When this adipose tissue organoid was implanted next to breast or pancreatic tumors in mice, it acted as a powerful metabolic sponge. By aggressively consuming local glucose and fats, the organoids starved the cancer, leading to a dramatic reduction in tumor volume by over 50%. To prove this was the mechanism, they showed the anti-cancer effect was completely erased when mice were fed a high-sugar or high-fat diet, confirming the engineered fat won the competition for nutrients. The success of this strategy, further validated using patient-derived tumor organoids, demonstrates how 3D models are pivotal in designing and testing next-generation cell therapies.
Pinpointing the Cause of Fatty Liver Disease
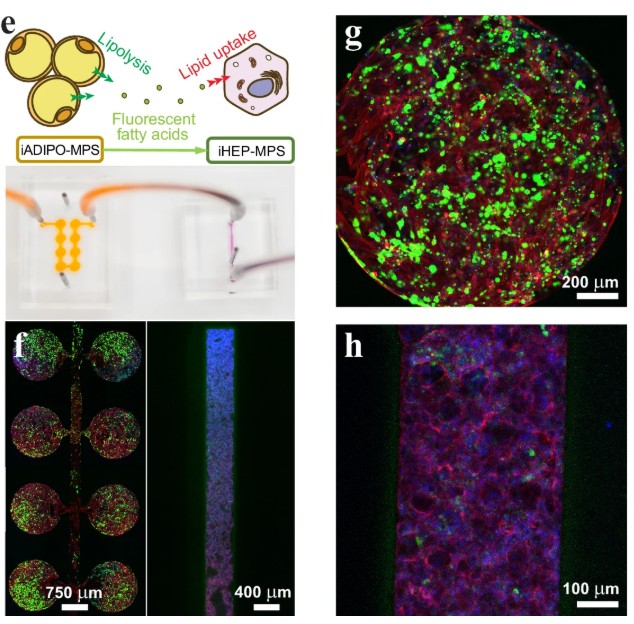
Obesity is tightly linked to serious conditions like type 2 diabetes and metabolic dysfunction-associated steatotic liver disease (MASLD), where the liver accumulates fat and stops responding properly to insulin. A central, unanswered question has long plagued researchers: what is the true trigger? Is it simply the expansion of white adipose tissue (fat mass), or is it the chronic inflammation within that fat, driven by immune cells, that poisons the liver? Answering this is incredibly difficult in humans or animal models because fat expansion and inflammation almost always occur together. This puzzle called for new approaches to methodologies capable of recreating the complex, multi-organ crosstalk between fat, liver, and the immune system in a controlled human model.
To solve this, researchers built a sophisticated multi-organ “adipose tissue on chip”. This microphysiological system (MPS) connected a chamber containing a 3D adipose tissue organoid with a second chamber containing liver cells. A key innovation was using cells (adipocytes, hepatocytes, and macrophages) all derived from the same line of human induced pluripotent stem cells (iPSCs), eliminating any genetic variability. First, they tested the “fat mass” hypothesis by increasing the ratio of fat to liver cells. Only at an extreme, physiologically unrealistic 30:1 ratio did they observe insulin resistance. However, when they introduced proinflammatory macrophages to the adipose tissue organoid to simulate obesity-driven inflammation, the results were dramatic. The inflamed fat released a cocktail of fatty acids and inflammatory signals that caused the connected liver cells to rapidly accumulate fat and become severely insulin-resistant. This elegantly demonstrated that fat tissue inflammation, not its mass alone, is the primary driver of liver damage, a finding made possible only by using these advanced, interconnected organoids.
Engineering Fat for the Future of Meat
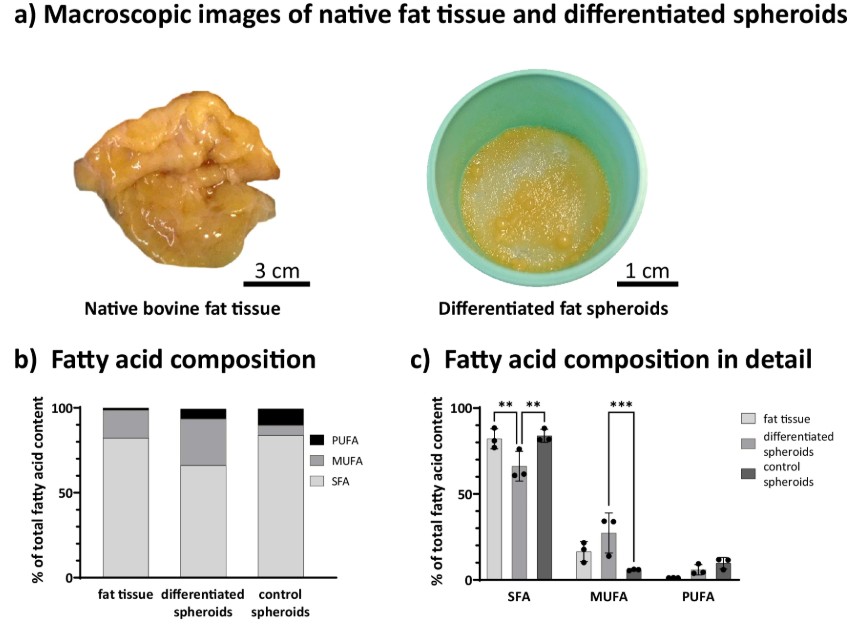
For cultured meat to move from a scientific curiosity to a supermarket staple, it must win over consumers. This means replicating the taste, texture, and juiciness of conventional meat that are qualities largely defined by fat. However, producing large quantities of animal fat tissue in a lab is a major bottleneck. Traditional 2D cell culture methods are inefficient, costly, and difficult to scale up for food production. This critical challenge has spurred the search for new approach methodologies that can generate structured fat tissue efficiently, creating a product that is both economically viable and appealing to the palate.
This study presents a clever solution by creating “living building blocks” of fat. Researchers took adipose-derived stem cells from cattle and, instead of growing them on flat surfaces, cultured them in dynamic suspension. This forced the cells to self-assemble into dense, 3D organoids. Using a simplified one-step protocol, they successfully differentiated these clusters into a functional adipose tissue organoid, packed with lipid droplets. These fat organoids proved to be remarkably robust and viable, even in large-scale dynamic cultures. In a key demonstration of their utility, the team used these organoids as a component in a 3D-printable, edible bioink made of gellan gum. They successfully bioprinted grid-like structures, showing the fat organoids survived the process and were evenly distributed. A final analysis of the fatty acid composition revealed that the cultured fat had significantly less saturated fat and more healthy monounsaturated fats than native bovine fat, offering a tantalizing glimpse into creating potentially healthier meat products.
A New Switch to Grow More 'Good' Fat
Brown adipose tissue (BAT), or “good fat,” is our body’s natural furnace, burning calories to generate heat. Having more of it is linked to better metabolic health and a lower risk of obesity. For years, therapeutic strategies have focused on activating the brown fat we already have. However, a far more powerful approach would be to increase the actual number of these fat-burning cells, a goal that has remained elusive. The main signaling molecule, cAMP, was thought to work primarily through one pathway (PKA) to trigger fat burning. This left a major gap in understanding how to encourage the growth of new brown fat, requiring new approaches and methodologies to uncover alternative pathways.
This study identifies a different signaling protein, EPAC1, as a master regulator of brown fat growth. Pharmacologically activating EPAC1 in mice led to a significant increase in BAT mass, boosted energy expenditure, and protected them from diet-induced obesity. The authors discovered that while the classic PKA pathway activates energy burning, the EPAC1 pathway specifically drives the proliferation (or cell division) of brown fat precursor cells. To confirm these findings in a human-relevant model, they turned to a sophisticated 3D culture system. Using human induced pluripotent stem cells, they grew a brown fat organoid, creating a miniature, functional version of the tissue. When they activated EPAC1 in this adipose tissue organoid, they observed a significant increase in both cell proliferation and the differentiation of these new cells into mature, energy-burning adipocytes. This pivotal result, achieved using organoids, demonstrates that EPAC1 is a key switch for expanding our body’s fat-burning capacity.
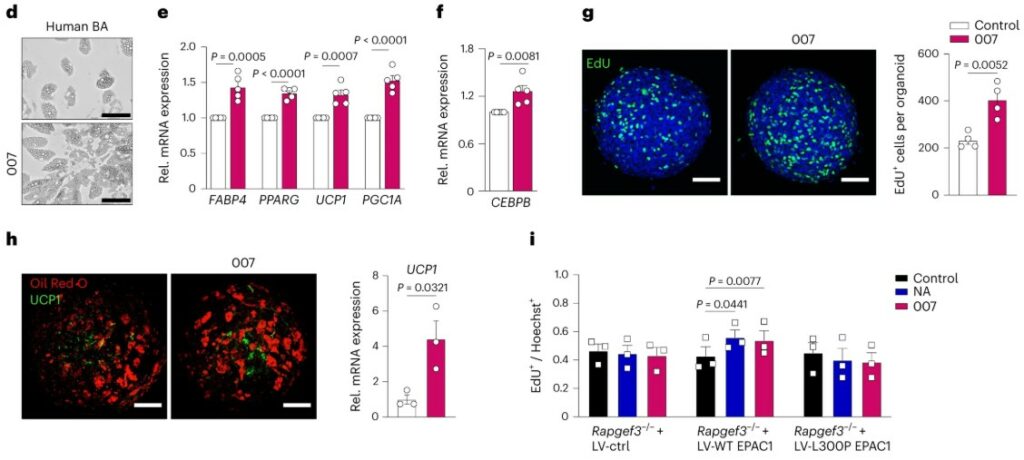
Building an Immune-Competent Fat Organoid
Adipose tissue is far more than just a storage depot for fat; it’s a dynamic immunological organ where fat cells (adipocytes) constantly communicate with resident immune cells, especially macrophages. This crosstalk is central to metabolic health, and its breakdown is a key driver of inflammation in obesity and type 2 diabetes. However, studying this interaction in a dish has been a major hurdle. Most in vitro models, including early adipose tissue organoid systems, lacked these crucial immune cells, forcing researchers to add them in from external sources. This created an urgent need for new approach methodologies to develop a 3D model that includes the native immune cell populations from the very beginning.
This study successfully created the first self-organizing adipose tissue organoid that contains its own resident immune cells. The researchers started with the stromal vascular fraction (SVF) from mouse adipose tissue, which is a complex mixture of adipocyte precursors and various immune cells. Using a simple scaffold-free method in ultra-low attachment plates, they allowed the SVF to self-assemble into a 3D structure. After differentiation, these organoids contained mature, lipid-storing adipocytes alongside a stable population of resident macrophages and mast cells. Remarkably, these immune cells survived and were maintained throughout the culture period without any added growth factors, suggesting the 3D microenvironment itself provided the necessary support. Furthermore, a detailed lipid analysis revealed that the lipid profile of the 3D organoid was significantly more similar to native adipose tissue than that of traditional 2D cell cultures, confirming it as a more physiologically relevant model.
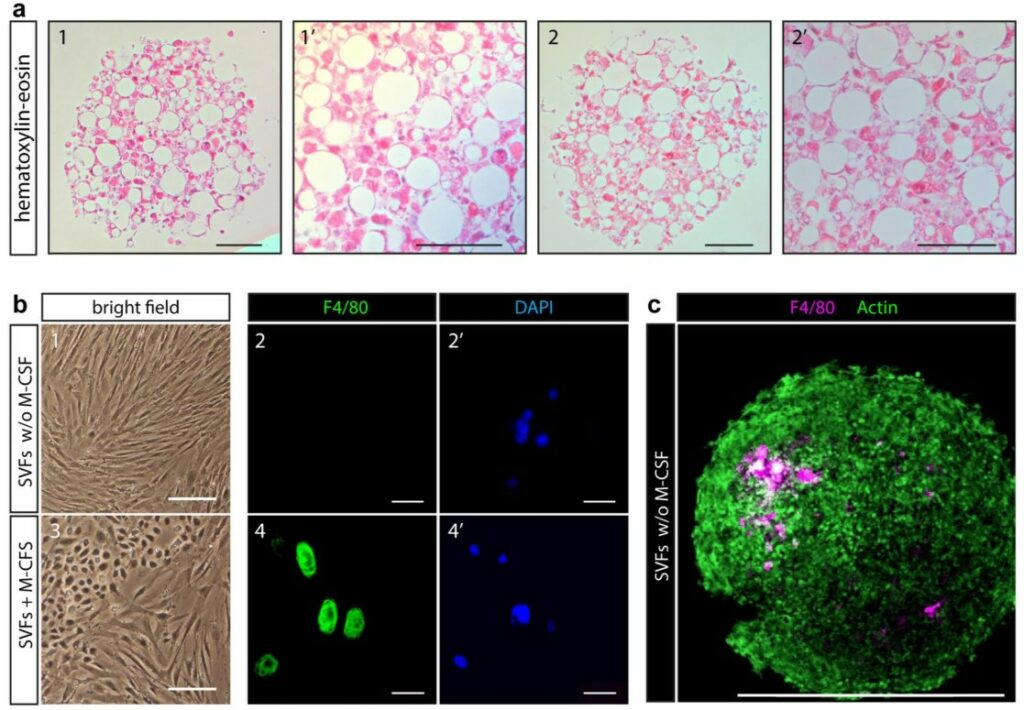
Modeling Fatty Liver Disease on a Chip
Nonalcoholic fatty liver disease (NAFLD) is a silent epidemic linked to obesity, yet its exact cause remains a “chicken-or-egg” puzzle. While inflammation is known to be a key player, it’s unclear if inflammatory signals directly damage the liver or if they first disrupt fat tissue, which then releases harmful factors that poison the liver. Animal models often fail to replicate human metabolism, and standard cell cultures can’t model the crucial crosstalk between organs. This gap has highlighted the need for new approach methodologies that can recreate human multi-organ interactions to untangle the complex web of signals that drive liver disease.
To address this, scientists developed a “human-on-a-chip” platform connecting two distinct organ modules: one with human liver cells (hepatocytes) and another with an adipose tissue model. They exposed this system to media simulating healthy, diabetic, obese, and inflammatory conditions. The inflammatory state was mimicked by adding the cytokine TNF-α. In a crucial control experiment using only liver cells, TNF-α alone failed to cause significant fat accumulation (steatosis). However, in the interconnected adipose tissue on chip, the same TNF-α treatment triggered a dramatic increase in liver fat. This elegantly demonstrated that TNF-α’s damaging effect on the liver is indirect; it first acts on the fat tissue, causing it to release harmful signals that then travel to and injure the liver cells. This key insight was only possible because this advanced multi-organ platform, going beyond simple organoids, could successfully model the systemic communication between fat and liver.
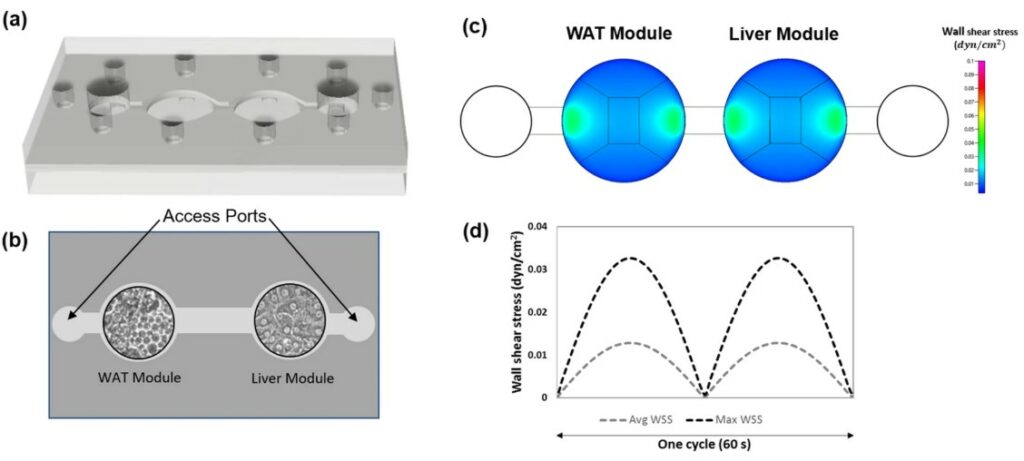
A Long-Term Home for Human Fat Cells
To accurately study human obesity and diabetes, scientists need to work with the most relevant cells: mature human white adipocytes. However, these cells are notoriously difficult to work with. Once removed from the body, they are extremely fragile, buoyant, and tend to lose their mature characteristics (a process called de-differentiation) within just a few days in a standard lab dish. This short lifespan has been a major barrier to conducting the long-term studies needed for drug development and understanding chronic metabolic diseases. This critical limitation highlighted the need for new approach methodologies to create a stable microenvironment that could keep these vital cells alive and functional for extended periods.
This study (Rogal et al, Advanced Science) introduces a groundbreaking adipose tissue on chip designed specifically to solve this problem. The researchers built a microfluidic device with specialized tissue chambers to house 3D microtissues made from primary human mature adipocytes. By embedding the fragile cells in a protective collagen hydrogel before loading them into the chip, they successfully overcame the issues of buoyancy and fragility. The platform features a vasculature-like microchannel that perfuses the tissue with nutrients, separated by a porous membrane that shields the cells from damaging fluid shear forces. The outcome was a resounding success: the adipose tissue on chip maintained the mature human fat cells in a viable and highly functional state for over a month. The team validated this by showing the cells maintained their structure, actively took up and released fatty acids, secreted key metabolites, and responded correctly to a drug stimulus, proving the platform’s utility for long-term mechanistic and pharmaceutical research.
A Breast Cancer Coculture Model in a Dish
The link between obesity and a poor prognosis for breast cancer is well-established, but the precise biological reasons are still murky. A leading hypothesis is that the metabolic dialogue between mammary adipocytes (M-Ads) (the fat cells surrounding a tumor) and the cancer cells themselves goes awry in obesity. Specifically, it’s thought that an increased transfer of fuel in the form of free fatty acids (FFAs) from M-Ads might supercharge tumor growth. However, a major obstacle to testing this has been the lack of a suitable model; like other mature adipocytes, human M-Ads are notoriously difficult to keep alive in traditional 2D lab cultures, where they quickly die. This limitation has driven the need for new approach methodologies to create a 3D environment that can support these delicate cells and enable the study of their crosstalk with cancer.
This study introduces a novel 3D culture model specifically designed to overcome these challenges. The researchers embedded primary human M-Ads, isolated from both lean and obese patients, into a supportive fibrin matrix. This simple yet effective method preserved the adipocytes’ integrity, morphology, and metabolic function for up to five days. A critical finding was that maintaining the cells in a medium with physiological glucose levels (5 mM) was essential to prevent a loss of their normal lipolytic function. Using this validated 3D culture, they set up a coculture experiment with breast cancer cells in a transwell system. This setup allowed them to demonstrate that FFAs were indeed transferred from the M-Ads to the cancer cells. Most importantly, this transfer was significantly amplified when using M-Ads derived from obese patients, providing direct evidence that obesity enhances this nurturing metabolic pathway. The success of this 3D model, which functions as a simplified adipose tissue organoid, provides a powerful new tool to dissect the fat-cancer dialogue.
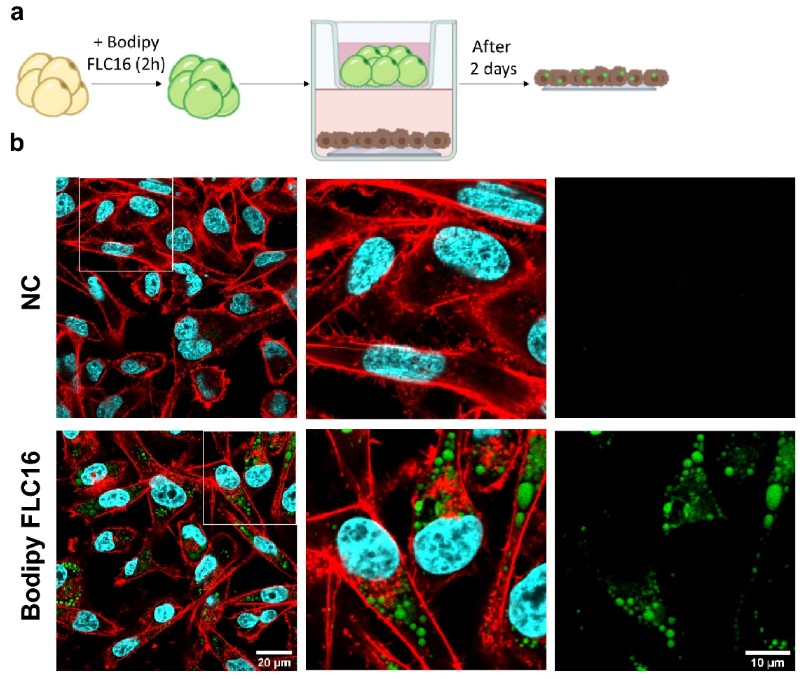
Mimicking Weight Gain in a Dish
Adipocyte enlargement, or hypertrophy, is the primary way our fat tissue expands during adult weight gain and is a key feature of obesity. This cellular enlargement is strongly linked to insulin resistance and metabolic disease. However, studying this process in a lab has been incredibly difficult. Traditional 2D cell cultures produce human adipocytes that are small and contain many tiny lipid droplets (multilocular), a poor imitation of the large, single-droplet (unilocular) fat cells found in the body. This has prevented researchers from accurately modeling how weight gain leads to cellular dysfunction, creating a major need for new approaches to build a more realistic human adipocyte model.
This study (Ioannidou et al, The Journal of Physiology) introduces an innovative 3D culture model called HUVAS (human unilocular vascularized adipocyte spheroids). Researchers hypothesized that recreating the vascular “niche” that fat cells grow in would improve their development. They took human adipose progenitor cells and co-cultured them with endothelial cells in ultra-low attachment plates, allowing them to self-assemble into an adipose tissue spheroid. The crucial step was embedding this spheroid in a supportive matrix (Matrigel), which prompted the endothelial cells to form a network of vascular sprouts. This vascular niche was a game-changer; the adipocytes within the HUVAS spheroid developed significantly larger lipid droplets and a more unilocular morphology than cells in other models. To mimic weight gain, they “fattened” these spheroids by adding a lipid mixture to the culture media. This successfully induced cellular hypertrophy, and critically, also triggered key features of adipocyte dysfunction, including insulin resistance and altered hormone secretion.
Building Functional, Vascularized Beige Fat
Activating the body’s energy-burning “beige” adipose tissue is a highly promising therapeutic strategy to combat obesity and type 2 diabetes. However, progress is severely hampered by the lack of relevant human models. While some 3D models exist, they often fail to include a crucial component: a built-in vascular network. Blood vessels are not just plumbing; they are essential for delivering nutrients, removing waste, and providing the developmental signals that fat tissue needs to mature and function properly. This has created a major challenge, requiring new approach methodologies to engineer a beige fat model that is not only functional but also pre-vascularized, making it suitable for both lab research and potential therapeutic transplantation.
This study (Escudero et al, Advanced Science) outlines an innovative bottom-up engineering strategy to build a functional and vascularized human beige adipose tissue organoid. The process starts with a heterogeneous mix of cells called the stromal vascular fraction (SVF), isolated from human white fat, which contains both adipose and endothelial progenitors. These cells are first aggregated into a spheroid. The key step was embedding this spheroid into a precisely tuned gelatin-methacryloyl (GelMA) hydrogel while simultaneously inhibiting the TGF-β signaling pathway. This specific biomechanical and chemical microenvironment guided the cells to self-organize into a complex structure. The resulting adipose tissue organoid successfully mimicked native beige fat, featuring both mature beige adipocytes and a self-assembled vascular network. Functionality was confirmed by showing the organoid could express the key thermogenic protein UCP1 when stimulated, increase its mitochondrial respiration, and secrete the appropriate signaling molecules known as batokines. The approach was also scalable, allowing the assembly of multiple units into centimeter-scale micro-tissues.
FAQ
Progress in treating metabolic diseases has been limited. This limitation is caused by a reliance on models that do not faithfully represent human adipose tissue biology. Traditional 2D cultures and animal models are often used. These systems fail to capture complex cellular crosstalk or metabolic dysfunction accurately. Therapeutic responses are also not well-represented. Over the past few years, major advances have been made by adopting human-derived 3D adipose tissue models. These models, which include organoids and organ-on-chip systems, provide higher fidelity. They are also encouraged by regulatory agencies. This shift allows for more predictive research.
Yes, strong encouragement for these models is provided at the regulatory and industrial level. Agencies such as the FDA and NIH have explicitly promoted these New Approach Methodologies (NAMs). This promotion is part of a roadmap. The goal is to reduce reliance on animal testing. Organoids and organ-on-chip systems are mentioned by these agencies. They are seen as validated tools. It is believed their use can accelerate translation. Preclinical drug evaluation may also be improved. This strategy is being adopted widely. Researchers are integrating these in vitro models with clinical data.
A strategy has been developed to create a competitor for nutrients consumed by cancer cells. Standard white fat cells were engineered using CRISPR. This engineering caused the cells to over-express UCP1, which transformed them into highly metabolic “beige” adipocytes. These cells were then grown into a stable, 3D adipose tissue organoid. This organoid arrangement was suitable for transplantation. When implanted next to tumours in mice, it functioned as a metabolic sponge. Local glucose and fats were aggressively consumed by the organoid. This action starved the cancer. A large reduction in tumour volume (over 50%) was observed.
A sophisticated multi-organ “adipose tissue on chip” was built. This microphysiological system connected a three-dimensional adipose tissue organoid with a separate chamber containing liver cells. A central question about metabolic dysfunction-associated steatotic liver disease (MASLD) was being investigated: is the trigger fat mass or fat inflammation? When proinflammatory macrophages were introduced to the adipose organoid, the results were clear. This simulated inflammation related to obesity. The inflamed fat released a mixture of fatty acids and inflammatory signals. This release caused the connected liver cells to rapidly accumulate fat. The liver cells also became severely insulin-resistant. It was demonstrated that fat tissue inflammation, not just its mass, is the main cause of the liver damage.
Replicating the taste and texture of conventional meat is a challenge for the cultured meat industry. These qualities are largely defined by fat. Producing animal fat tissue in a lab efficiently is a major bottleneck. A solution was presented using “living building blocks.” Adipose-derived stem cells from cattle were cultured in dynamic suspension. This process forced the cells to self-assemble into dense 3D organoids. These clusters were differentiated into functional adipose tissue. The resulting fat organoids were shown to be stable and viable. They were successfully used as a component in a 3D-printable, edible bioink, surviving the printing process.
A signaling protein, EPAC1, has been identified as a regulator of brown fat growth. Brown adipose tissue (BAT), or “good fat,” is known to burn calories. Increasing the number of these cells is a powerful therapeutic goal. Previously, the main signaling molecule, cAMP, was thought to work mainly through the PKA pathway to activate energy burning. This new study discovered that the EPAC1 pathway specifically causes the proliferation, or cell division, of brown fat precursor cells. These findings were confirmed in a human-relevant model. A 3D brown fat organoid was grown from human induced pluripotent stem cells. When EPAC1 was activated in this organoid, a large increase in cell proliferation was observed.
Yes, the first self-organising adipose tissue organoid that contains its own resident immune cells has been successfully created. Adipose tissue is understood to be an active immunological organ. However, studying the crosstalk between fat and immune cells has been difficult because most models lack these cells. In this study, the stromal vascular fraction (SVF) from mouse tissue was used. This SVF, a complex mixture of precursor and immune cells, was allowed to self-assemble in a scaffold-free method. The resulting 3D organoids contained mature, lipid-storing adipocytes. A stable population of resident macrophages and mast cells was also present, surviving throughout the culture period without added growth factors.
Mature human white adipocytes are notoriously difficult to work with. Once removed from the body, the cells are extremely fragile and buoyant. They also tend to lose their mature characteristics, a process called de-differentiation, within just a few days in a standard culture dish. This short lifespan is a major barrier to long-term studies. An “adipose tissue on chip” was introduced to solve this problem. In this microfluidic device, 3D microtissues of primary adipocytes are embedded in a protective collagen hydrogel. This embedding overcomes fragility. The tissue is perfused by a vasculature-like microchannel, which is separated by a porous membrane. This design shields cells from damaging fluid shear forces, maintaining them in a viable, functional state for over a month.
How can the process of weight gain be reproduced in a lab model?
The lack of a built-in vascular network has limited progress in beige fat models. Blood vessels are not just for transport; they are necessary for delivering nutrients and providing developmental signals that fat tissue needs to mature. An engineering strategy was used to build a functional and vascularized human beige adipose tissue organoid. The process started with a heterogeneous mix of cells (SVF), which contains both adipose and endothelial progenitors. This cell mix was aggregated into a spheroid. The spheroid was then embedded into a precisely tuned GelMA hydrogel, and a specific signaling pathway (TGF-β) was inhibited. This microenvironment guided the cells to self-organise into a complex arrangement, featuring mature beige adipocytes and a self-assembled vascular network.




