In our previous article, we outlined the main in vitro liver models, immortalized cell lines such as HepG2 and HepaRG, alongside primary hepatocytes, and discussed their respective advantages, applications, and limitations. These systems provide the foundation for preclinical liver research, but their predictive value is often constrained by the shortcomings of conventional two-dimensional (2D) culture. Rapid dedifferentiation, loss of polarity, and declining metabolic activity limit their ability to capture complex, long-term liver functions. In this follow-up, we compare 2D and 3D liver culture systems and examine how 3D culture enhance the physiological relevance of these established models for drug discovery, toxicology, and disease modeling.
Learn more about our ready to use human liver organoid models.
Limitations of 2D cell culture
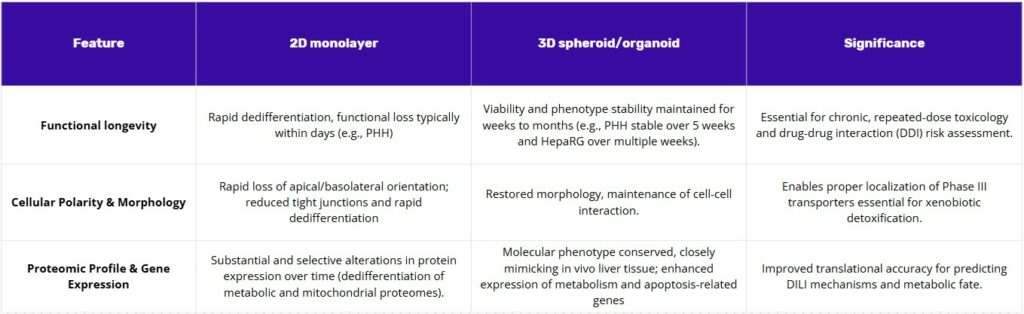
2D monolayers have been indispensable for short-term assessments, but they are intrinsically limited by rapid functional decline. Primary human hepatocytes undergo dedifferentiation within days, losing polarity and the structural features needed for bile canaliculi formation, alongside steep drops in cytochrome P450 activity. Proteomic analyses show that this collapse reflects a selective remodeling of the mitochondrial and metabolic proteomes, which explains the diminished metabolic competence and the failure to capture chronic or bioactivation-dependent mechanisms of drug-induced liver injury. Immortalized lines are not exempt: in 2D, HepG2 retains inherently low xenobiotic-metabolizing capacity, and even differentiated HepaRG can display variability and functional drift over time. Collectively, these shortcomings limit the translational value of 2D systems, particularly for prolonged or repeated-dose paradigms.
How 3D improves liver culture systems
The superior performance of 3D culture models is a direct result of their ability to restore physiological structure. The re-establishment of cell-cell and cell-matrix interactions in a 3D environment restores cellular polarity and phenotype, which in turn leads to the sustained expression of key functional genes, such as CYPs, and enhances long-term viability. This causal relationship is the fundamental scientific principle that explains why 3D models are more predictive than 2D cultures.
Notably for liver research, spheroids demonstrate significantly enhanced liver-specific functions compared to their 2D counterparts, including improved albumin and urea secretion and sustained cytochrome P450 (CYP) activity for weeks or months. This long-term viability is particularly beneficial for studying slowly metabolized compounds or for conducting repeated-dose toxicity studies that reveal cumulative or delayed effects, which are not possible in short-lived 2D systems. Spheroids also exhibit a greater sensitivity to hepatotoxicants like acetaminophen, making them a superior model for drug-induced hepatotoxicity testing(1).
The next frontier in this field is the development of multicellular systems. The incorporation of non-parenchymal cells (NPCs) like Kupffer macrophages, stellate cells, and endothelial cells into 3D co-cultures allows for the modeling of complex multicellular responses, including inflammation, fibrosis, and immune-mediated liver injury. This approach acknowledges that liver function is a synergistic process that extends beyond the hepatocytes alone, providing a more complete and accurate understanding of drug-induced liver injury (DILI) and disease progression.
What 3D culture brings to liver models
HepG2
The HepG2 cell line, derived from a human hepatocellular carcinoma, remains prevalent in initial screenings due to its high proliferative capacity and ease of maintenance. While severely compromised in terms of drug-metabolizing capacity and expression of key hepatic proteins common to hepatocytes, its function is improved substantially in 3D culture. In HepG2 spheroids, xenobiotic metabolism and liver functions are boosted: CYP1A1 mRNA increases by ~10-fold with corresponding activity gains, and albumin production per cell over 7 days rises to ~2.5× that of 2D, even when albumin mRNA returns to baseline, indicating architecture-dependent functional output. Crucially, when spheroids are replated back into 2D, the enhanced CYP1A1 and albumin functions are lost, underscoring that these improvements are maintained by the 3D context rather than media alone. Transcriptome profiling further shows that 3D architecture reprograms HepG2 toward liver-specific function (upregulating xenobiotic and lipid-metabolism genes such as CYP1A1, AKR1C1, EPHX1, GSTA1/GCLM), whereas 2D monolayers preferentially upregulate ECM/adhesion/cytoskeletal programs (e.g., COL1A1, CSPG2, CDH1, CLDN6) highlighting that structure directs function in this model(2,3).
The most valuable contribution of 3D HepG2 spheroids lies in their ability to transition toward a more quiescent, tissue-like state. Unlike 2D monolayers, HepG2 spheroids exhibit a time-dependent suppression of cell division, leading to the accumulation of cells in the G0/G1 phase of the cell cycle, which is characteristic of mature, non-proliferating hepatocytes. This morphological and proliferation shift is accompanied by the downregulation of proliferation markers and time-dependent deregulation of genes encoding hepatic markers and metabolic phase I/II enzymes. Furthermore, liver functions such as albumin secretion and CYP activity show rapid improvement in specific HepG2 variants when cultured in a 3D environment(2).
One other critical benefit of the HepG2 spheroid model is the improved accuracy of toxicity assessment, largely by preventing the false positives common in 2D systems. Spheroids exhibit a considerably increased resistance to toxic substances compared to 2D cells. This heightened resistance is a direct consequence of the pronounced intracellular junctions and dense extracellular matrix developed in 3D organization. These structures physically influence xenobiotic transport by decreasing drug penetration into the spheroid core. The inherent lack of 3D organization in 2D HepG2 models thus frequently leads to an overestimation of cellular toxicity. By providing a more physically robust and stable microenvironment, 3D HepG2 spheroids yield a more reliable initial toxicity filter, better aligning the in vitro concentration of toxicity with concentrations required in vivo.
The 3D culture configuration also enables these cells to maintain enhanced function for longer, making the HepG2 spheroid model useful for predicting long-term hepatotoxicity via repeated dosing, despite its overall lower metabolic profile compared to HepaRG or PHH(4).
HepaRG Cells
HepaRG cells, derived from a human liver progenitor cell line, are valued for their ability to differentiate into a mixture of hepatocyte-like cells and biliary epithelial cells, exhibiting a phenotype closer to primary human hepatocytes (PHH) than HepG2 cells. Differentiated HepaRG cells maintain a stable phenotype and constitutive CYP450 expression over several weeks in culture, positioning them as a robust intermediate model.(5,6).
The shift of HepaRG cells from 2D monolayer to 3D spheroid culture results in dramatic, quantifiable improvements in metabolic function, making them far more predictive for metabolism-dependent toxicity and drug-drug interaction (DDI) studies.
Specific data illustrates this functional leap, particularly for Phase I enzyme activity: 3D HepaRG spheroids exhibit constitutive CYP1A2 activity (measured by acetaminophen formation rates from phenacetin) of 51.8±12.0 pmol/min-million cells. In contrast, 2D HepaRG cultures measured only 7.69±0.354 pmol/min-million cells. This means the activity in the 3D spheroids was approximately 6.7-fold higher than in the 2D cultures.
This level of constitutive CYP1A2 activity in 3D HepaRG spheroids is highly significant, as it approaches the metabolic capacity of PHH models. The 51.8 pmol/min-million cells activity is comparable to the 25th percentile of cryopreserved PHH suspensions (47.1 pmol/min-million cells), providing a stable, metabolically competent platform suitable for industrial screening(7).
Furthermore, 3D HepaRG spheroids show enhanced overall expression of genes related to not only drug and glucose metabolism but also lipid metabolism (e.g., fatty acid and triglyceride synthesis, apolipoproteins APOC1 and APOE). They demonstrate higher activities for Phase I and II enzymes (including Glutathione-S-transferases and Aldo keto reductases) compared to 2D cultures, retaining these functions for longer periods(8,9).
The 3D configuration enhances the cells’ response to prototypical CYP inducers. Gene expressions for key enzymes like CYP1A2, CYP2B6, and CYP3A4, following treatment with omeprazole, phenobarbital, and rifampicin, were significantly higher in 3D HepaRG spheroids compared to 2D conditions. This robust inducibility confirms that the nuclear receptor pathways, such as the Pregnane X Receptor (PXR) and Constitutive Androstane Receptor (CAR), which are essential for DDI risk assessment, are highly functional and regulated within the 3D tissue architecture(8).
The physical cues provided by the spheroid architecture (cell-cell contact and polarity restoration) act as a powerful maturation signal, leading to more consistently high-performing cells. This enhanced function is critical because HepaRG differentiation protocols are known to be complex, potentially leading to variability in reported data. The robust boost in function conferred by the 3D architecture helps override inherent functional variability, resulting in a more reliable model for high-confidence industrial screens. Consequently, HepaRG spheroids are considered superior to HepG2 spheroids in functional protein expression and sensitivity for detecting metabolically activated toxicants
Primary Human Hepatocytes (PHH)
PHH remain the benchmark for in vitro hepatic studies, owing to their unmatched physiological fidelity. They retain the complete set of drug-metabolizing enzymes, nuclear receptors, and transporters necessary for pharmacokinetics, toxicology, and disease modeling. However, in conventional 2D culture they undergo rapid dedifferentiation, losing polarity and cytochrome P450 activity within days. As a result, they fail to adequately capture chronic, low-dose injury or the sustained bioactivation processes that underlie many cases of drug-induced liver injury (DILI).
3D culture systems have decisively improved the utility of PHHs. In spheroids, metabolic activities remain high and stable over extended periods, with far less decline than observed in matched 2D sandwich cultures from the same donor. At the proteomic level, 3D PHHs conserve the molecular phenotype of native liver tissue, avoiding the selective remodeling that undermines 2D models. Functionally, they can capture the staged progression of hepatotoxicity in a time-resolved manner: an early drop in albumin or urea production is followed by the release of clinical injury markers such as ALT and AST, and finally by overt cell death measured through ATP content. This temporal cascade mirrors clinical liver injury and enables repeated low-dose exposure studies that reveal delayed or idiosyncratic toxicity. Moreover, among widely used in vitro models, PHH spheroids show the strongest correlation with in vivo CYP inducibility and can discriminate between closely related compounds, such as differentiating the high toxicity of troglitazone from the safer profile of pioglitazone(10,11).
Recent work has extended these capabilities even further. Igarashi et al. (2025) reported protocols to generate long-term organoids from adult hepatocytes, sustaining differentiation and functional bile canalicular networks by modulating Wnt and STAT3 signaling. These organoids preserved major metabolic functions and resisted ductal metaplasia, offering a scalable and durable system for toxicology and metabolic disease studies(12). Complementing this, Reza et al. (2025) introduced multi-zonal liver organoids that recreate the porto-central axis, with zone 1– and zone 3–like hepatocyte compartments supporting distinct functions such as ammonia detoxification, urea cycling, and xenobiotic metabolism. Such zonated models enable sophisticated mechanism-of-action studies, advanced disease modeling, and late-stage drug assessments where spatial heterogeneity of hepatic response is critical(13).
Strategy for drug screening (what model for what)
A practical screening strategy moves from simple, high-throughput tests toward more complex, physiologically relevant models. Typically, this means beginning with a quick, reliable initial screen, then advancing to a cell line capable of drug metabolism and interaction testing, and finally using primary human cells for longer-term liver injury assessment. Three-dimensional culture is preferable throughout because it helps cells maintain their function, restore their normal organization, and better predict human responses.
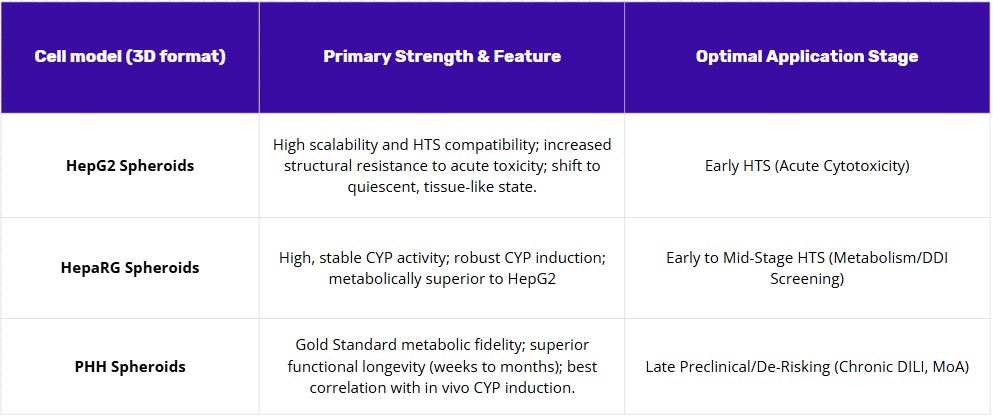
HepG2 spheroids work well for initial screening. They’re stable and easy to work with, making them useful for quick toxicity tests and short repeat-dose studies. Growing them in 3D creates more realistic conditions—nutrients and test compounds diffuse through the spheroid rather than hitting cells all at once, which reduces the misleading positives common in flat cultures. Since HepG2 cells have limited metabolic capacity, this stage focuses on direct toxicity rather than effects that depend on drug metabolism.
HepaRG spheroids handle the middle phase: metabolism and drug interaction studies. HepaRG cells already metabolize drugs better than HepG2 in standard cultures, but the 3D format further boosts their enzyme activity (including key enzymes like CYP1A2), maintains a broader range of metabolic functions, and preserves their response to enzyme-inducing drugs (such as rifampicin or phenobarbital). They also maintain proper directional transport for weeks, making them suitable for studying compounds that are toxic only after metabolism or that interact with drug transporters. They require more effort than HepG2 but are still far easier to use than primary cells.
For final assessment of chronic liver injury risk, primary human hepatocyte (PHH) spheroids are the most relevant model. The aim here is to generate reliable evidence under conditions that mimic patient exposure: extended treatment at realistic doses that can reveal progressive damage; declining liver function first, followed by elevated damage markers, and eventually cell death. PHH spheroids maintain high metabolic activity for weeks and show drug responses that most closely match human clinical data. Because of donor variation and cost, it makes sense to use them only after the earlier screening stages have narrowed down the candidate list.
Conclusion
In vitro liver models remain indispensable for drug discovery and toxicology, each providing distinct strengths: HepG2 for robust screening, HepaRG for metabolic competence, and primary hepatocytes for benchmark human relevance. However, their predictive power is limited by the constraints of standard 2D culture, where rapid loss of function undermines long-term studies. Recognizing these challenges is critical for selecting the right model at the right stage of research. Continued innovation, particularly in culture technologies, offers the opportunity to preserve function and improve translational accuracy, ensuring safer and more effective pathways from preclinical studies to clinical outcomes.
References
- Mizoi K, Arakawa H, Yano K, Koyama S, Kojima H, Ogihara T. Utility of Three-Dimensional Cultures of Primary Human Hepatocytes (Spheroids) as Pharmacokinetic Models. Biomedicines. 23 sept 2020;8(10):374.
- Štampar M, Breznik B, Filipič M, Žegura B. Characterization of In Vitro 3D Cell Model Developed from Human Hepatocellular Carcinoma (HepG2) Cell Line. Cells. 28 nov 2020;9(12):2557.
- Chang TT, Hughes-Fulford M. Monolayer and Spheroid Culture of Human Liver Hepatocellular Carcinoma Cell Line Cells Demonstrate Distinct Global Gene Expression Patterns and Functional Phenotypes. Tissue Eng Part A. mars 2009;15(3):559‑67.
- Yang S, Ooka M, Margolis RJ, Xia M. Liver three-dimensional cellular models for high-throughput chemical testing. Cell Rep Methods. mars 2023;3(3):100432.
- Mandon M, Huet S, Dubreil E, Fessard V, Le Hégarat L. Three-dimensional HepaRG spheroids as a liver model to study human genotoxicity in vitro with the single cell gel electrophoresis assay. Sci Rep. 22 juill 2019;9(1):10548.
- Lőrincz T, Deák V, Makk-Merczel K, Varga D, Hajdinák P, Szarka A. The Performance of HepG2 and HepaRG Systems through the Glass of Acetaminophen-Induced Toxicity. Life. 21 août 2021;11(8):856.
- Ramaiahgari SC, Waidyanatha S, Dixon D, DeVito MJ, Paules RS, Ferguson SS. Three-Dimensional (3D) HepaRG Spheroid Model With Physiologically Relevant Xenobiotic Metabolism Competence and Hepatocyte Functionality for Liver Toxicity Screening. Toxicol Sci. 1 nov 2017;160(1):189‑90.
- Takahashi Y, Hori Y, Yamamoto T, Urashima T, Ohara Y, Tanaka H. 3D spheroid cultures improve the metabolic gene expression profiles of HepaRG cells. Biosci Rep. 1 juin 2015;35(3):e00208.
- Gunness P, Mueller D, Shevchenko V, Heinzle E, Ingelman-Sundberg M, Noor F. 3D Organotypic Cultures of Human HepaRG Cells: A Tool for In Vitro Toxicity Studies. Toxicol Sci. mai 2013;133(1):67‑78.
- Cox CR, Lynch S, Goldring C, Sharma P. Current Perspective: 3D Spheroid Models Utilizing Human-Based Cells for Investigating Metabolism-Dependent Drug-Induced Liver Injury. Front Med Technol. 30 nov 2020;2:611913.
- Bell CC, Dankers ACA, Lauschke VM, Sison-Young R, Jenkins R, Rowe C, et al. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicol Sci. 1 avr 2018;162(2):655‑66.
- Igarashi R, Oda M, Okada R, Yano T, Takahashi S, Pastuhov S, et al. Generation of human adult hepatocyte organoids with metabolic functions. Nature [Internet]. 16 avr 2025 [cité 13 mai 2025]; Disponible sur: https://www.nature.com/articles/s41586-025-08861-y
- Reza HA, Santangelo C, Iwasawa K, Reza AA, Sekiya S, Glaser K, et al. Multi-zonal liver organoids from human pluripotent stem cells. Nature [Internet]. 16 avr 2025 [cité 13 mai 2025]; Disponible sur: https://www.nature.com/articles/s41586-025-08850-1
FAQ
Two-dimensional monolayers have been widely used for short-term tests. They are inherently limited by a rapid decline in function. For example, primary human hepatocytes undergo dedifferentiation within days. They lose their polarity and the structural features needed for bile canaliculi formation. Steep drops in cytochrome P450 activity are also observed. This functional collapse is shown by proteomic analyses to be a result of metabolic proteome remodelling. Because of these issues, the 2D systems fail to capture chronic mechanisms of drug-induced liver injury. Their translational value is limited, especially for prolonged or repeated-dose studies.
The higher performance of 3D culture models is a direct result of their ability to restore physiological structure. Cell-cell and cell-matrix interactions are re-established in this environment. This restoration leads to renewed cellular polarity and phenotype. In turn, the sustained expression of important functional genes, such as CYPs, is achieved. Long-term viability is also enhanced. Liver-specific functions are shown to be improved in spheroids. This includes better albumin and urea secretion. Sustained cytochrome P450 activity for weeks or months is also seen. This long-term viability allows for studies of slowly metabolised compounds.
The HepG2 cell line is common for initial screenings but has compromised metabolic capacity. Its function is greatly improved in 3D culture. In HepG2 spheroids, xenobiotic metabolism is boosted. CYP1A1 mRNA is reported to increase by about 10-fold, with corresponding activity gains. Albumin production per cell over seven days also rises. It is important that when spheroids are replated back into 2D, these enhanced functions are lost. This shows the improvements are maintained by the 3D context. The 3D architecture also reprograms HepG2 cells toward liver-specific functions. They shift to a more quiescent, tissue-like state, with more cells in the G0/G1 phase.
The 3D spheroid model improves the accuracy of toxicity assessment. This is largely achieved by preventing the false positives that are common in 2D systems. Spheroids exhibit a greatly increased resistance to toxic substances when compared to 2D cells. This heightened resistance is a direct result of the pronounced intracellular junctions. A dense extracellular matrix is also developed in the 3D organisation. These structures physically influence xenobiotic transport. They decrease drug penetration into the spheroid core. The inherent lack of this organisation in 2D models often leads to an overestimation of cellular toxicity. 3D HepG2 spheroids provide a more reliable initial toxicity filter.
HepaRG cells are derived from a human liver progenitor cell line. They are recognised for their ability to differentiate into a mixture of two cell types. These are hepatocyte-like cells and biliary epithelial cells. This mixture results in a phenotype that is closer to primary human hepatocytes (PHH) than HepG2 cells. In culture, differentiated HepaRG cells maintain a stable phenotype. They also show constitutive CYP450 expression over several weeks. These characteristics position them as a stable intermediate model. They are more complex than HepG2 cells but easier to use than primary cells.
Yes, the shift of HepaRG cells from 2D to 3D spheroid culture results in large, measurable improvements in metabolic function. Specific data shows this functional leap. For Phase I enzyme activity, 3D HepaRG spheroids exhibit constitutive CYP1A2 activity of 51.8±12.0 pmol/min-million cells. In contrast, 2D HepaRG cultures measured only 7.69±0.354 pmol/min-million cells. This means the activity in 3D spheroids was about 6.7-fold higher. This level of activity is noteworthy, as it approaches the metabolic capacity of PHH models. The 3D model provides a stable, metabolically competent platform suitable for industrial screening.
PHH remain the benchmark for in vitro hepatic studies. This is due to their high physiological fidelity. They retain the complete set of drug-metabolising enzymes, nuclear receptors, and transporters. These are all necessary for pharmacokinetic and toxicology modelling. Their main limitation is seen in conventional 2D culture. They undergo rapid dedifferentiation. Polarity and cytochrome P450 activity are lost within a few days. As a result, 2D PHH models fail to adequately capture chronic, low-dose injury. They also cannot model the sustained bioactivation processes seen in many cases of drug-induced liver injury.
Three-dimensional culture systems have clearly improved the utility of PHHs. In spheroids, metabolic activities are kept high and stable over extended periods. There is far less functional decline than is observed in 2D sandwich cultures from the same donor. At the proteomic level, 3D PHHs conserve the molecular phenotype of native liver tissue. This conservation avoids the selective remodelling that weakens 2D models. Functionally, they can capture the staged progression of hepatotoxicity in a time-resolved manner. This mirrors clinical liver injury. It enables repeated low-dose exposure studies that can reveal delayed toxicity.
Recent work has extended the capabilities of PHH models. Protocols have been reported to generate long-term organoids from adult hepatocytes. These models sustain differentiation and functional bile canalicular networks. This is achieved by modulating Wnt and STAT3 signaling. These organoids preserve major metabolic functions. In addition, multi-zonal liver organoids have been introduced. These newer models recreate the porto-central axis of the liver. They establish zone 1– and zone 3–like hepatocyte compartments. These distinct zones support different functions, such as ammonia detoxification, urea cycling, and xenobiotic metabolism.
A practical screening strategy moves from simple tests toward more complex, physiologically relevant models. Three-dimensional culture is recommended throughout this process. The strategy begins with HepG2 spheroids for initial screening. They are stable and useful for quick toxicity tests. This stage focuses on direct toxicity, as these cells have limited metabolic capacity. Next, HepaRG spheroids are used for the middle phase. This stage assesses metabolism and drug interactions, as their 3D format boosts enzyme activity. For the final assessment of chronic liver injury risk, primary human hepatocyte (PHH) spheroids are the most relevant model. They are used last, after the candidate list is narrowed, due to donor variation and cost.