Introduction
Human vascular microphysiological systems (MPS) represent promising three-dimensional in vitro models of normal and diseased vascular tissue. These systems, which mimic essential functional components of organs and tissues, draw on breakthroughs in tissue engineering, microfluidics, and stem cell differentiation.

Vascular models for microvasculature and medium-sized arterioles have already been established. Key vascular system functions have been replicated, and stem cells have the ability to mimic hereditary disorders and population diversity in genes that may raise an individual’s risk of cardiovascular disease. These systems can be used to assess novel treatment alternatives. Learn more in the paper featured below.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The authors state that “Microphysiological systems (MPSs), based on microfabrication technologies and cell culture, can faithfully recapitulate the complex physiology of various tissues. However, 3D tissues formed using MPS have limitations in size and accessibility; their use in regenerative medicine is, therefore, still challenging.
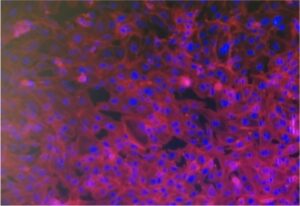
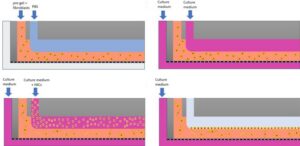
Here, an MPS-inspired scale-up vascularized engineered tissue construct that can be used in regenerative medicine is designed. Endothelial cell-laden hydrogels are sandwiched between two through-hole membranes. The microhole array in the through-hole membranes enables the molecular transport across the hydrogel layer, allowing long-term cell culture. 3D Microphysiological System Vascularized Tissue
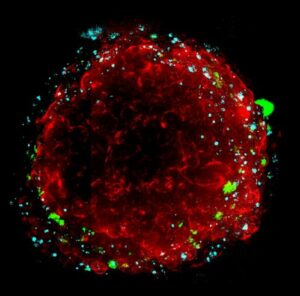
Furthermore, the time-controlled delamination of through-hole membranes enables the harvesting of cell-cultured hydrogel constructs without damaging the capillary network. Importantly, when the tissue constructs are implanted in a mouse ischemic model, they protect against necrosis and promoted functional recovery to a greater extent than implanted cells, hydrogels, and simple gel–cell mixtures.”.
References
Bang, S., Tahk, D., Choi, Y. H., Lee, S., Lim, J., Lee, S., Kim, B., Kim, H. N., Hwang, N. S., & Jeon, N. L. (2021). 3D Microphysiological System‐Inspired Scalable Vascularized Tissue Constructs for Regenerative Medicine. Advanced Functional Materials, 2105475. https://doi.org/10.1002/adfm.202105475
FAQ
Human vascular microphysiological systems (MPS) are considered beneficial three-dimensional in vitro models. They are used to represent normal and diseased vascular tissue. These systems are designed to reproduce the essential functional components of organs and tissues. Their development is based on progress in tissue engineering, microfluidics, and the differentiation of stem cells. Vascular models have already been established for microvasculature. Models for medium-sized arterioles also exist. Main functions of the vascular system have been replicated in these models. These systems provide a new way to study blood vessel tissues in a laboratory setting.
Stem cells can be used within vascular microphysiological systems. They possess an ability to reproduce hereditary disorders. Population diversity related to specific genes can also be represented. This is relevant for genes that might increase a person’s risk of developing cardiovascular disease. By incorporating stem cells with these genetic variations, more specific disease models can be created. These systems can then be applied to evaluate new treatment options. This provides a method for testing potential therapies on models that represent specific genetic conditions or population groups. This application is a main reason for using stem cells in these advanced models.
Microphysiological systems, which are based on microfabrication and cell culture, can faithfully reproduce the intricate physiology of various tissues. A drawback has been identified for 3D tissues formed using these standard MPS. These tissues have restrictions related to their size and their accessibility. Because of these issues, their application in regenerative medicine is still considered difficult. To address this, a new system was designed. It is an MPS-inspired, scaled-up, vascularized engineered tissue construct. This new construct was specifically developed for use in regenerative medicine. It is intended to overcome the size and access problems of previous models.
The new construct is designed with hydrogels containing endothelial cells. These hydrogels are positioned between two membranes that have through-holes. An array of microholes in these membranes permits molecular transport to move across the hydrogel layer. This design allows the cells to be cultured for an extended period. A specific feature of the system is the time-controlled delamination of the membranes. This process allows the cell-cultured hydrogel construct to be harvested. It is noted that this removal can be done without harming the capillary network that has formed inside the hydrogel. This harvesting ability is important for its application in regenerative medicine.