Introduction
Adipose tissue-breast cancer crosstalk is emerging as a key driver of tumor initiation, progression, and therapy resistance, linking epidemiological obesity signals to local, proximal interactions within the breast cancer tumor microenvironment. Here, we synthesize how dysfunctional adipocytes and adipose stromal cells fuel malignancy and how tumors reciprocally reprogram fat, then show how 3D in vitro models, including adipose tissue and breast cancer organoids co-culture bridge tumor modeling and drug discovery to target the adipose–tumor axis.
This article is a focus on cancer-associated adipocyte and a follow-up of our article on the influence of tumor microenvironment on breast cancer development that you can read here.
Learn more about our ready to use breast cancer organoid models.
Epidemiology and global evidence for adipose tissue–cancer interactions
There is now compelling epidemiological evidence that excess body fatness is causally linked to both cancer incidence and cancer‑associated mortality across multiple tumor types. The International Agency for Research on Cancer (IARC) concluded in 2016 that “excess body fatness” increases risk for at least 13 cancers, extending earlier lists to include gastric cardia, liver, gallbladder, pancreas, ovary and thyroid cancers, multiple myeloma, and meningioma, with consistent dose–response relationships for many of these sites(1). Echoing this, contemporary endocrine and oncology reviews emphasize that adipose tissue is a large, active endocrine organ whose dysfunction in obesity contributes to carcinogenesis systemically and locally(2,3).
For breast cancer specifically, higher post‑diagnosis adiposity is associated with worse outcomes. A comprehensive Global Cancer Update Program meta‑analysis (225 observational studies) reported that every 5 kg/m² increase in BMI after diagnosis confers a 10% higher breast cancer–specific mortality (RR 1.10, 95% CI 1.06–1.14) and a 7% higher all‑cause mortality (RR 1.07, 95% CI 1.05–1.10), supporting a probable causal relationship(4). Notably, similar positive associations were observed for waist circumference and waist‑hip ratio, underscoring that body fat distribution matters beyond BMI(2,4).
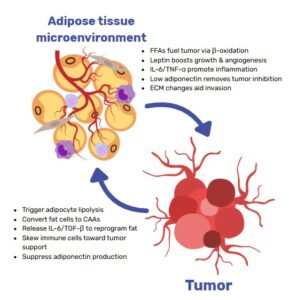
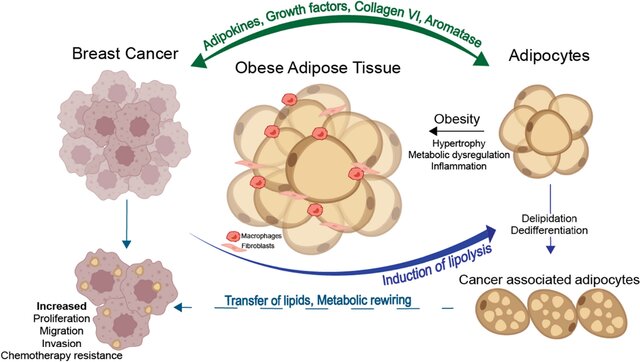
Mechanistically, dysfunctional adipose tissue in obesity is characterized by insulin resistance, chronic low‑grade inflammation, altered adipokine profiles (e.g. increased leptin, decreased adiponectin), elevated circulating glucose, insulin and free fatty acids, and extensive extracellular matrix remodeling that can systemically foster tumor growth, as shown for hepatocellular carcinoma and pancreatic ductal adenocarcinoma, among others(2,3,5). However, in Adipose tissue breast cancer biology, a unique layer of risk emerges because malignant epithelial cells reside within, or immediately adjacent to, dense mammary adipose depots. This spatial proximity enables intense, bidirectional paracrine, metabolic and mechanical crosstalk, in which adipocytes (and the broader stromal vascular fraction) become “cancer‑associated adipocytes” that fuel invasion, metabolic reprogramming and therapeutic resistance(2,3,5).
In what follows, we first recall the physiological roles of adipose tissue in normal breast development, then dissect the proximal interactions between breast tumors and adjacent fat, and finally discuss how advanced 3D in vitro systems (from breast cancer organoids and adipose tissue organoid co‑cultures to organ‑on‑chip tumor modeling) allow us to interrogate and therapeutically exploit this adipose‑enriched breast cancer tumor microenvironment.
Adipose tissue in normal breast development
Breast adipose tissue is indispensable for mammary morphogenesis and function. During puberty, adipocytes provide endocrine and paracrine cues such as adipokines (notably an increased leptin‑to‑adiponectin ratio), estrogens via stromal aromatase, and inflammatory mediators, that help activate the hypothalamic–pituitary–gonadal axis and drive ductal elongation and branching. In mouse models lacking white adipose tissue (A‑ZIP/F‑1), a rudimentary gland forms but fails to grow or branch properly, underscoring the trophic role of adipocytes in epithelial expansion.
During pregnancy and lactation, mammary adipocytes undergo intense lipolysis, releasing free fatty acids that are taken up and metabolically converted by mammary epithelial cells to sustain milk production; adipocytes can even dedifferentiate into precursor‑like cells and later re‑expand through hypertrophy during involution. Collectively, these data position adipocytes as dynamic regulators of mammary gland development, metabolism, and hormonal milieu across the life course(2).
Proximal interactions between breast cancer and adipose tissue
At the tumor/adipose tissue interface, breast cancer cells and adjacent adipocytes engage in bidirectional interactions. In 2D transwell co‑cultures, Balaban et al. showed that breast cancer cells actively deplete adipocyte triacylglycerol, import adipocyte‑derived free fatty acids (FFAs), and upregulate CPT1A and electron‑transport chain components to increase fatty‑acid oxidation and promote breast cancer cell proliferation and migration. These effects were attenuated when adipocyte lipolysis was inhibited(6). In a patient‑derived 3D biomimetic platform, Toyoda et al. studied direct lipid transfer from mature adipocytes to breast cancer cells, reinforcing that proximal metabolic crosstalk is a core feature of the breast cancer tumor microenvironment(7).
However, Adipose tissue–breast cancer crosstalk is not limited to FFA transfer for energy: adipocytes and adipose stromal cells (ASCs) support nearly all phases of tumor development including growth, invasion, dissemination, and therapy resistance via paracrine signaling (leptin, IL‑6, TNF‑α), extracellular matrix (ECM) remodeling, and metabolic provisioning (FFAs, lactate, ketone bodies). Lengyel et al. and Quail & Dannenberg review evidence that local white adipose tissue (WAT) becomes inflamed and fibrotic, with crown‑like structures, stromal infiltration, and increased aromatase, thereby potentiating proximal oncogenic signaling and invasion(5,8). Solsona‑Vilarrasa & Vousden further emphasize that dysfunctional WAT supplies altered adipokine gradients, FFAs, and ECM cues that directly reprogram nearby cancer cells and stromal populations to favor chemoresistance and immune evasion(3).
Crucially, the crosstalk is reciprocal. Tumor cells reprogram adipocytes into cancer‑associated adipocytes (CAAs), characterized by delipidation, pro‑inflammatory secretomes, and ECM‑remodeling activity. Notably, Zhu et al. provided genetic and single‑cell evidence that mammary tumor invasion can trigger adipocyte mesenchymal transition (AMT) with de‑differentiation process into myofibroblast‑ and macrophage‑like states that amplify stromal stiffness, inflammation, and pro‑tumorigenic signaling. These remodeled adipocyte-derived cells become functionally indistinguishable from tumor‑associated fibroblasts in their capacity to drive proliferation(9).
Collectively, proximal interactions encompass: (i) metabolic coupling (adipocyte lipolysis → tumor fatty‑acid oxidation [FAO]), (ii) phenotypic reprogramming of adipocytes to CAAs/AMT, (iii) ASC recruitment and ECM remodeling (fibrosis, collagen VI fragments, MMPs), and (iv) inflammation‑driven survival and drug tolerance. Targeting these local axes may be essential to overcome the adipose‑conditioned aggressiveness and chemoresistance of breast tumors.
How 3D in vitro models can capture the interplay between adipose tissue and breast cancer
Co‑culture systems, particularly 3D, offer an essential reductionist model to interrogate the local and proximal crosstalk between adipocytes (and ASC) and tumor cells without the confounding inputs of whole‑body metabolism, immunity, or pharmacokinetics. In the context of adipose tissue breast cancer biology, they allow precise control over cell composition, matrix and stiffness, intercellular distance, and microfluidics hard to tune in vivo.
Organoid co-culture examples to study local interactions.
- Rebeaud et al. established a 3D fibrin matrix model for primary human mammary adipocytes that preserves viability and lipolytic ability, enabling quantitative tracking of FFA transfer to breast cancer cells, which was amplified under obese conditions(10).
- Watzling et al. used direct 3D co‑spheroids (breast cancer cells + ASCs/adipocytes) to show that cell–cell contact and 3D architecture alone up‑regulate CCL5/CCR1, functionally enhancing TNBC cell migration. This elegant demonstration shows that mechanistically relevant signals can be missed in 2D settings(11).
- Blyth et al. pushed complexity further with a penta‑culture obesity-associated breast cancer model (tumor cells, myoepithelial cells, macrophages, fibroblasts, adipocytes), capturing the inflamed adipose border and revealing obesity‑linked paclitaxel resistance and greater metformin sensitivity, underscoring the translational value of multi-lineage organoid research for tumor modeling and drug testing(12).
- Jeong et al. introduce a bioprinted, patient-specific breast cancer–adipose composite tissue in which inter-tissue distance is tunable, enabling parametric evaluation of adipokine gradients, invasion, and drug efficacy(13).
Organ‑on‑chip (OOC) platforms (adding systemic features).
Microphysiological systems integrate perfusion, endothelial barriers, immune compartments, and controlled endocrine/metabolic inputs, allowing researchers to bridge local adipocyte–tumor co-culture phenomena with systemic‑like gradients (glucose, insulin, leptin) and pharmacological exposures. Such organ-on-chip devices thereby complement organoids: organoids allow deciphering cellular-resolution mechanisms, while OOCs simulate hemodynamics, transport, and kinetics that condition those mechanisms in vivo.
Resources
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body Fatness and Cancer — Viewpoint of the IARC Working Group. N Engl J Med. 25 août 2016;375(8):794‑8.
- Brown KA, Scherer PE. Update on Adipose Tissue and Cancer. Endocr Rev. 9 nov 2023;44(6):961‑74.
- Solsona‐Vilarrasa E, Vousden KH. Obesity, white adipose tissue and cancer. FEBS J. mai 2025;292(9):2189‑207.
- Chan DSM, Vieira R, Abar L, Aune D, Balducci K, Cariolou M, et al. Postdiagnosis body fatness, weight change and breast cancer prognosis: Global Cancer Update Program (CUP global) systematic literature review and meta‐analysis. Int J Cancer. 15 févr 2023;152(4):572‑99.
- Lengyel E, Makowski L, DiGiovanni J, Kolonin MG. Cancer as a Matter of Fat: The Crosstalk between Adipose Tissue and Tumors. Trends Cancer. mai 2018;4(5):374‑84.
- Balaban S, Shearer RF, Lee LS, Van Geldermalsen M, Schreuder M, Shtein HC, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. déc 2017;5(1):1.
- Toyoda Y, Celie KB, Xu JT, Buro JS, Jin J, Lin AJ, et al. A 3-Dimensional Biomimetic Platform to Interrogate the Safety of Autologous Fat Transfer in the Setting of Breast Cancer. Ann Plast Surg. avr 2018;80(4):S223‑8.
- Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol. mars 2019;15(3):139‑54.
- Zhu Q, Zhu Y, Hepler C, Zhang Q, Park J, Gliniak C, et al. Adipocyte mesenchymal transition contributes to mammary tumor progression. Cell Rep. sept 2022;40(11):111362.
- Rebeaud M, Bouche C, Dauvillier S, Attané C, Arellano C, Vaysse C, et al. A novel 3D culture model for human primary mammary adipocytes to study their metabolic crosstalk with breast cancer in lean and obese conditions. Sci Rep. 22 mars 2023;13(1):4707.
- Watzling M, Klaus L, Weidemeier T, Horder H, Ebert R, Blunk T, et al. Three-Dimensional Breast Cancer Model to Investigate CCL5/CCR1 Expression Mediated by Direct Contact between Breast Cancer Cells and Adipose-Derived Stromal Cells or Adipocytes. Cancers. 5 juill 2023;15(13):3501.
- Blyth RRR, Laversin SA, Foxall RB, Savva C, Copson E, Cutress RI, et al. Development and characterisation of a novel 3D in vitro model of obesity-associated breast cancer as a tool for drug testing. Npj Breast Cancer [Internet]. 30 mai 2025 [cité 25 juill 2025];11(1). Disponible sur: https://www.nature.com/articles/s41523-025-00766-3
- Jeong W, Han J, Choi J, Kang HW. Embedded Bioprinting of Breast Cancer–Adipose Composite Tissue Model for Patient-Specific Paracrine Interaction Analysis. Adv Healthc Mater. 1 janv 2025;14(3):2401887.
FAQ
Yes, epidemiological information shows that excess body fatness is causally linked to a higher incidence of at least 13 types of cancer. Adipose tissue is understood to be an active endocrine organ. In obesity, its dysfunction can contribute to carcinogenesis. For breast cancer, higher adiposity after diagnosis is associated with worse outcomes. A large meta-analysis reported that for every 5 kg/m² increase in BMI, there is a 10% higher breast cancer-specific mortality. A 7% higher all-cause mortality was also noted. Body fat distribution, such as waist circumference, also matters, not just BMI.
Malignant epithelial cells in the breast are located within or next to dense mammary adipose depots. This spatial proximity allows for intense, bidirectional communication between the cell populations. This local interaction is a distinct layer of risk in adipose tissue breast cancer biology. Because of this closeness, adipocytes (fat cells) can be changed. They become "cancer-associated adipocytes". These changed cells then provide substances that promote tumour invasion. This local interaction also supports metabolic reprogramming of the tumour. Therapeutic resistance is also supported by these interactions.
Breast adipose tissue is needed for mammary morphogenesis and function. During puberty, adipocytes supply endocrine and paracrine cues. These cues include adipokines, such as an increased leptin-to-adiponectin ratio, and estrogens from stromal aromatase. These signals help activate the hypothalamic–pituitary–gonadal axis. They also assist ductal elongation and branching. During pregnancy and lactation, mammary adipocytes undergo intense lipolysis. This process releases free fatty acids. These are then taken up and metabolically converted by mammary epithelial cells to sustain milk production. Adipocytes are shown to be dynamic regulators of the mammary gland.
Breast cancer cells are shown to actively deplete adipocyte triacylglycerol, which is their stored fat. This was observed in 2D transwell co-culture experiments. The cancer cells import the free fatty acids (FFAs) that are derived from the adipocytes. They also upregulate CPT1A and components of the electron-transport chain. This action increases their ability to use fatty-acid oxidation. This process was found to support breast cancer cell proliferation and migration. When adipocyte lipolysis (the breakdown of fat) was inhibited, these effects were lessened. This proximal metabolic communication was reinforced by a 3D biomimetic platform. Direct lipid transfer from mature adipocytes to breast cancer cells was studied.
The communication is not limited to energy transfer. Adipocytes and adipose stromal cells (ASCs) support many phases of tumour development. This includes growth, invasion, and dissemination. Paracrine signaling is one method of support, using molecules like leptin, IL-6, and TNF-α. Extracellular matrix (ECM) remodeling is another way support is given. Metabolic provisioning of FFAs, lactate, and ketone bodies also occurs. Local white adipose tissue (WAT) can become inflamed and fibrotic. This state is marked by crown-like structures and increased aromatase, which potentiates oncogenic signaling. This environment also supports chemoresistance and immune evasion.
Yes, the communication is reciprocal. Tumour cells are shown to reprogram adipocytes. The fat cells are turned into cancer-associated adipocytes, or CAAs. These CAAs are characterized by delipidation, meaning they lose their stored fat. They also develop pro-inflammatory secretomes and activity related to ECM-remodeling. Genetic and single-cell evidence has been provided. This evidence showed that mammary tumour invasion can trigger adipocyte mesenchymal transition (AMT). This is a process of de-differentiation. The adipocytes change into myofibroblast- and macrophage-like states. These remodeled cells then increase stromal stiffness, inflammation, and pro-tumorigenic signaling. They become functionally similar to tumour-associated fibroblasts.
Co-culture systems, especially those in 3D, are used as reductionist models. They are employed to interrogate the local and proximal communication between adipocytes and tumour cells. These models operate without the confounding inputs of whole-body metabolism or immunity. Precise control over the experimental conditions is allowed in these in vitro systems. This includes control over cell composition, the matrix, and its stiffness. Intercellular distance and microfluidics can also be managed. These are factors that are difficult to tune in a living organism. For example, a 3D fibrin matrix model preserved adipocyte viability. This preservation allowed for the quantitative tracking of FFA transfer.
Direct 3D co-spheroids were used in one study. These spheroids contained breast cancer cells mixed with adipose stromal cells (ASCs) or adipocytes. The experiment showed that cell–cell contact and the 3D architecture alone were sufficient to cause changes. An upregulation of CCL5 and CCR1 was observed. This upregulation functionally increased the migration of triple-negative breast cancer (TNBC) cells. This demonstration was noteworthy. It showed that mechanistically relevant signals can be missed when using 2D settings. The 3D arrangement provided a different set of cues. These cues led to a different cellular behaviour.
A more intricate model was developed, referred to as a penta-culture. This model was designed to represent obesity-associated breast cancer. It included tumour cells, myoepithelial cells, macrophages, fibroblasts, and adipocytes. The inflamed adipose border was successfully captured by this system. The model also revealed resistance to paclitaxel that was linked to obesity. Greater sensitivity to metformin was also observed. This work indicates the translational value of multi-lineage organoid research. Such models are useful for tumour modeling. They are also useful for drug testing.
Microphysiological systems, or organ-on-chip (OOC) platforms, add other features. Perfusion, endothelial barriers, immune compartments, and controlled endocrine or metabolic inputs are integrated. These additions permit researchers to connect local adipocyte–tumour co-culture phenomena with systemic-like gradients. These gradients can include glucose, insulin, or leptin. Pharmacological exposures can also be simulated. Such devices are seen as a complement to organoids. Organoids are suited for deciphering cellular-resolution mechanisms. In contrast, OOCs simulate the hemodynamics, transport, and kinetics that condition those mechanisms within a living system.