What Does Adipose Tissue Do?
Adipose tissue is more than a fat storage site, it’s a dynamic endocrine organ central to metabolic health. It stores excess energy as triglycerides and releases it as fatty acids during fasting, while secreting hormones like leptin and adiponectin that regulate appetite, insulin sensitivity, and inflammation. Adipose tissue also responds to environmental stimuli such as diet, temperature, and stress, adapting its function accordingly. When this system is overwhelmed or dysregulated, it contributes directly to metabolic diseases like obesity, insulin resistance, and type 2 diabetes, making it a key target in drug discovery and therapeutic development.
Types of Adipose Tissue: Function and Location
There are two main types of adipose tissue: white (WAT) and brown (BAT). WAT primarily stores energy, while BAT burns it to generate heat through non-shivering thermogenesis. WAT is further divided into subcutaneous adipose tissue (SAT) (found beneath the skin) and visceral adipose tissue (VAT) (located around abdominal organs). SAT is generally protective, acting as a buffer against ectopic fat accumulation. In contrast, VAT is more metabolically active and closely linked to insulin resistance and cardiovascular risk. Depot-specific differences in inflammation, lipolysis, and expandability shape their distinct roles in metabolic health and disease(1).
The Function of White Adipose Tissue in Energy Storage
White adipose tissue (WAT) is the principal caloric reservoir, capturing excess plasma glucose and free fatty acids and re-esterifying them into triglycerides under the control of lipogenic enzymes and the sympathetic nervous system(2). During fasting or adrenergic stress, adipose-triglyceride-lipase and hormone-sensitive-lipase mobilize these stores to fuel peripheral tissues, illustrating WAT’s bidirectional energy buffering role. Yet it is widely accepted now that WAT is not just an energy reservoir, its unilocular adipocytes, stromal-vascular cells and resident immune cells secrete a rich cocktail of adipokines, including leptin, adiponectin, resistin, visfatin and hundreds of exosomal microRNAs, that modulate appetite, insulin action, vascular tone and even cognition. Obesity drives adipocyte hypertrophy, ECM stiffening and macrophage infiltration, shifting this secretome toward pro-inflammatory TNF-α, IL-6 and MCP-1 and precipitating systemic insulin resistance(3). Therapeutically, enhancing healthy WAT expansion (via selective PPARγ agonism), improving blood flow with incretin analogues and inducing beige conversion with cold, β3-agonists or FGF21 aim to restore its buffering and endocrine functions while preventing ectopic lipid spill-over.
Learn more about our ready to use Adipose organoid plate.
How Adipose Tissue Drives Metabolic Disease
The dysfunction of adipose tissue is not a passive consequence of obesity; it is a central driver of metabolic disorders. Obesity-related disorders such as insulin resistance, type 2 diabetes, fatty liver disease, and cardiovascular complications are increasingly recognized as originating from intrinsic defects in the function, distribution, and adaptability of adipose depots.
One of the earliest and most critical dysfunctions is the loss of adipose tissue expandability. In healthy individuals, the body accommodates excess energy through hyperplasia, the generation of new, small adipocytes that remain insulin-sensitive. This process preserves tissue flexibility and metabolic homeostasis. However, when progenitor cell capacity is limited, due to genetics, aging, inflammation, or environmental insults, excess lipids are shunted into existing adipocytes, causing them to hypertrophy. These hypertrophic cells become mechanically and metabolically stressed, promoting inflammation, lipotoxicity, and insulin resistance(2).
Hypertrophic adipocytes exhibit disrupted endocrine function. They secrete reduced levels of beneficial adipokines like adiponectin while releasing pro-inflammatory cytokines such as TNF-α, IL-6, and MCP-1. These cytokines attract immune cells, particularly macrophages, that further amplify inflammation through a self-sustaining feedback loop. This inflammatory shift is not only local but also systemic, affecting insulin signaling in the liver, muscle, and pancreas(3).
Depot location plays a critical role in metabolic health. Subcutaneous adipose tissue (SAT) is generally protective, expanding through hyperplasia and exhibiting lower inflammation. In contrast, visceral adipose tissue (VAT) is more lipolytically active and prone to immune cell infiltration. Rather than free fatty acids alone, current evidence points to VAT-derived inflammatory cytokines (such as IL-6, IL-1β, and RBP4) as key drivers of metabolic dysfunction. These cytokines reach the liver via the portal circulation, where they activate stress pathways like JNK and NF-κB, impairing insulin signaling and promoting hepatic insulin resistance. This chronic inflammatory signaling, rather than lipid overflow alone, now appears to underlie the harmful impact of visceral fat on systemic metabolism(4).
When adipose depots reach their storage limit, lipid overflow leads to ectopic fat deposition in non-adipose tissues like the liver (leading to non-alcoholic fatty liver disease), muscle (causing insulin resistance), and pancreas (impairing insulin secretion). These tissues are not equipped to safely store triglycerides, and the accumulation of lipid intermediates, such as diacylglycerols and ceramides, disrupts intracellular signaling, mitochondrial function, and insulin action. This phenomenon, known as lipotoxicity, is a key trigger of organ dysfunction in metabolic disease(5).
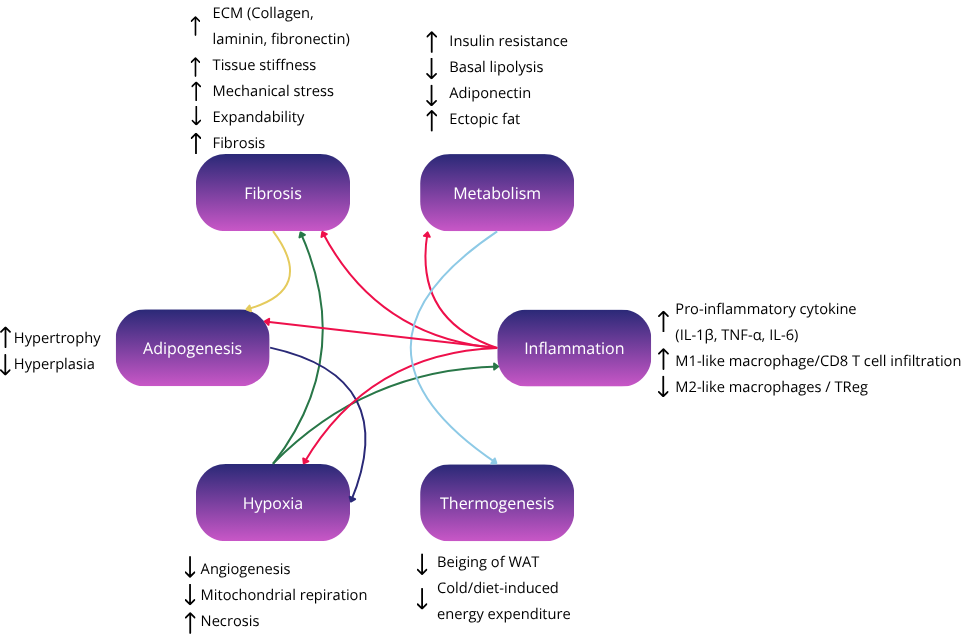
Another major pathological feature is tissue hypoxia and fibrosis. As adipocytes enlarge, their oxygen demand increases while the local vasculature fails to keep pace. The resulting hypoxia stabilizes hypoxia-inducible factor-1 alpha (HIF-1α), which promotes fibrotic remodeling rather than new blood vessel formation. The excess extracellular matrix (ECM), primarily composed of collagens, laminins, and fibronectin, creates a stiffened environment that impedes lipid mobilization, nutrient exchange, and adipocyte communication. Fibrosis also traps immune cells within the tissue, exacerbating local inflammation and worsening insulin resistance.
Importantly, this cycle of hypertrophy → hypoxia → fibrosis → inflammation is self-reinforcing. Fibrosis constrains further expansion, pushing lipids toward ectopic sites. Inflammatory cytokines inhibit adipogenesis and promote senescence among adipocyte progenitor cells, reducing the ability of fat tissue to renew and adapt. These senescent cells, in turn, secrete additional inflammatory and fibrotic signals, a pattern termed the senescence-associated secretory phenotype (SASP).
The immune landscape of adipose tissue also transforms in obesity. In lean states, anti-inflammatory cells like regulatory T cells (Tregs), eosinophils, and M2-like macrophages help maintain homeostasis. Obesity skews this balance toward pro-inflammatory M1-like macrophages, Th1 cells, and cytotoxic T cells, which further inhibit insulin signaling and support a fibrotic microenvironment.
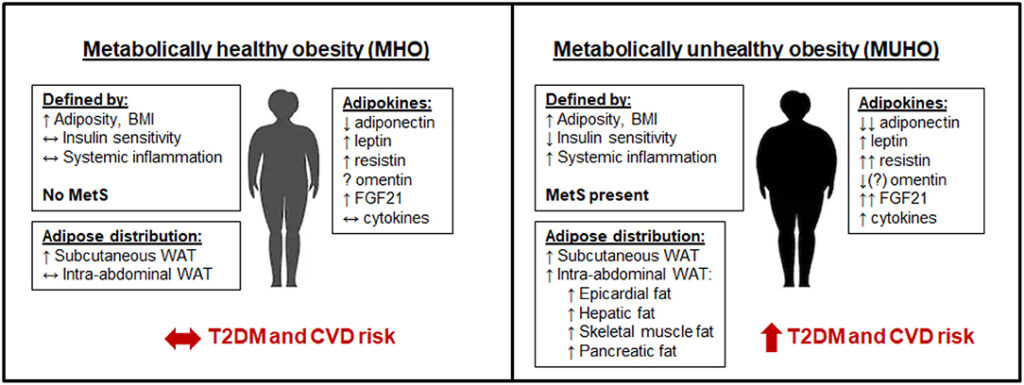
Thus, the development of metabolic disorders can be understood not merely as a matter of “too much fat,” but as a breakdown in the tissue’s ability to adapt and function as an endocrine organ. The balance between healthy expansion and pathological remodeling determines whether fat acts as a metabolic buffer or as a source of systemic dysfunction. This is particularly illustrated by the concept of metabolically healthy obese in comparison to metabolically unhealthy lean/obese individuals(6).
In summary, adipose tissue drives metabolic disease through several converging mechanisms(2):
- Limited expandability leading to unhealthy hypertrophy
- Depot-specific risk with VAT driving inflammation and lipotoxicity
- Ectopic fat deposition and lipid overflow damaging non-adipose organs
- Chronic inflammation and immune dysregulation
- Hypoxia-induced fibrosis and senescence
- Loss of endocrine homeostasis
Understanding and targeting these mechanisms is essential not only for treating obesity, but also for preventing and reversing its downstream metabolic complications. It’s also why physiologically relevant in vitro models of adipose tissue (such as organoids and organs-on-chips) are becoming indispensable tools in preclinical research.
Therapeutic Strategies Targeting Adipose Tissue
Given the central role of adipose tissue in metabolic disease, therapeutic strategies increasingly focus on restoring healthy fat function, not simply reducing fat mass. One foundational approach is improving adipose tissue expandability. Drugs like thiazolidinediones (TZDs), which activate PPAR-γ, promote the formation of new, small adipocytes through hyperplasia(7). While TZDs can increase overall fat mass, they redistribute lipids to safer subcutaneous depots and improve insulin sensitivity by reducing lipotoxicity in non-adipose organs.
Incretin-based therapies, such as GLP-1 receptor agonists (e.g., semaglutide) and dual GIP/GLP-1 agonists (e.g., tirzepatide), are also effective. They reduce appetite, body weight, and particularly visceral fat, leading to improved glycaemic control and cardiovascular outcomes. These agents are rapidly becoming first-line treatments for both obesity and type 2 diabetes(8).
Another promising avenue is targeting brown and beige adipocyte thermogenesis. Unlike white fat, brown fat dissipates energy as heat via uncoupling protein-1 (UCP1). Beige adipocytes, which can emerge within white fat depots in response to cold or β-adrenergic stimulation, offer an inducible thermogenic mechanism. Compounds that activate BAT or promote WAT “beiging”, such as FGF21 analogues, β3-adrenergic agonists, or cold mimetics, have shown potential to increase energy expenditure and improve insulin sensitivity. However, translating these findings to humans remains a challenge due to interspecies differences in BAT abundance and responsiveness(9).
Anti-fibrotic and pro-angiogenic therapies aim to reverse the mechanical and oxygen limitations of hypertrophic adipose tissue. For example, miRNA are studied to modulate ECM secretion and stiffening through key signaling pathways and thus alleviating adipose tissue fibrosis(10).
through key signaling pathways miRNA are studied to modulate ECM secretion and stiffening, thus
Immunomodulatory approaches are also gaining traction. Chronic inflammation in adipose tissue contributes to insulin resistance, and targeting inflammatory pathways (e.g., TNF-α, IL-1β, or JNK signaling) may help restore metabolic balance. Likewise, eliminating senescent cells in adipose tissue, using senolytic drugs, can rejuvenate adipose progenitor pools and improve adipogenesis in aged or obese individuals.
Lastly, cell-based therapies such as the transplantation of healthy adipocytes or adipose-derived stem cells are being explored in experimental models. These strategies aim to replace or reprogram dysfunctional fat, restore endocrine function, and improve metabolic health systemically(11).
Together, these diverse interventions highlight the therapeutic value of targeting adipose tissue function, not just size, as a core strategy against metabolic disease.
From Animal Models to Organoids: Evolving Tools for Adipose Tissue Research
For decades, our understanding of adipose tissue and metabolic disease has relied heavily on animal models, especially genetically modified mice. Mouse models have been instrumental in uncovering key mechanisms (such as the role of leptin, insulin resistance, or adipokine signaling). Diet-induced obesity (DIO) models and knockout strains continue to offer insights into systemic metabolic dysfunction. However, mice differ significantly from humans in adipose distribution, thermogenic activity, immune landscape, and drug metabolism, which limits the translation of findings into effective human therapies.
In vitro, researchers have long used preadipocyte cell lines like 3T3-L1 (mouse) and SGBS (human) cells to study adipogenesis and adipocyte biology. These models are reproducible and convenient but lack the cellular complexity, 3D architecture, and physiological cues found in native adipose tissue. They often fail to capture depot-specific differences (e.g., visceral vs. subcutaneous), immune interactions, or the dynamic response to mechanical and hormonal signals.
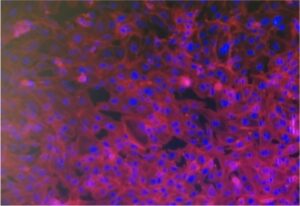
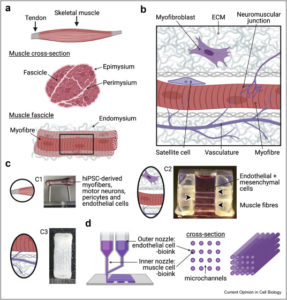
Recognizing these limitations, the field is rapidly moving toward 3D culture systems and microphysiological models. Adipose tissue organoids, derived from primary stromal vascular fractions or induced pluripotent stem cells (iPSCs), can self-organize into spheroids containing mature adipocytes, immune cells, and vascular-like structures. These organoids exhibit functional features such as lipid storage, adipokine secretion, and even fibrosis and inflammation under stress, offering a more faithful representation of human adipose physiology and pathology.
Complementing these are organ-on-chip platforms, which place adipose tissue (often alongside liver, muscle, or pancreas) within microfluidic devices that replicate blood flow, mechanical stress, and inter-organ communication. These systems allow real-time monitoring of lipid metabolism, insulin sensitivity, and cytokine profiles in response to therapeutic compounds.
As the pharmaceutical industry looks to reduce reliance on animal testing and improve translational accuracy, these advanced models represent a promising shift. They align with the 3Rs principle (Replacement, Reduction, Refinement) and provide high-content, human-relevant platforms for adipose tissue preclinical testing. The integration of 3D models, especially adipose tissue organoids and organ-on-chip systems, is now paving the way for more predictive drug discovery pipelines and a deeper understanding of how fat contributes to, or protects against, metabolic disease(12–15).
Conclusion
Adipose tissue is not merely a passive fat reservoir but a metabolically dynamic organ that influences the onset and progression of major diseases like type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease. Its ability, or failure, to store lipids safely, communicate with other organs, and maintain hormonal and immune balance makes it a central player in metabolic health. Importantly, not all fat is created equal: visceral and subcutaneous depots differ dramatically in their disease associations, and features like hypertrophy, fibrosis, inflammation, and hypoxia determine whether adipose tissue protects or harms.
As therapies increasingly aim to restore healthy adipose tissue function rather than simply reduce fat mass, there is a growing need for more predictive and human-relevant preclinical models. Adipose organoids and organ-on-chip systems now offer powerful platforms to replicate depot-specific biology, model disease mechanisms, and screen drug candidates with greater fidelity than traditional models.
For biotech innovators, researchers, and developers, integrating adipose-focused testing into early-stage pipelines is essential. Understanding how fat works, breaks down, and responds to therapy may be the key to unlocking the next generation of effective treatments for metabolic disease.
Resources
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. janv 2010;11(1):11‑8.
- Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. févr 2022;185(3):419‑46.
- Henninger AMJ, Eliasson B, Jenndahl LE, Hammarstedt A. Adipocyte Hypertrophy, Inflammation and Fibrosis Characterize Subcutaneous Adipose Tissue of Healthy, Non-Obese Subjects Predisposed to Type 2 Diabetes. Sun Q, éditeur. PLoS ONE. 22 août 2014;9(8):e105262.
- Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev. déc 2012;13(S2):30‑9.
- Trouwborst I, Bowser SM, Goossens GH, Blaak EE. Ectopic Fat Accumulation in Distinct Insulin Resistant Phenotypes; Targets for Personalized Nutritional Interventions. Front Nutr. 4 sept 2018;5:77.
- Chait A, Den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 25 févr 2020;7:22.
- Basak S, Murmu A, Matore BW, Roy PP, Singh J. Thiazolidinedione an auspicious scaffold as PPAR-γ agonist: its possible mechanism to Manoeuvre against insulin resistant diabetes mellitus. Eur J Med Chem Rep. août 2024;11:100160.
- Zheng Z, Zong Y, Ma Y, Tian Y, Pang Y, Zhang C, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther. 18 sept 2024;9(1):234.
- Thyagarajan B, Foster MT. Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm Mol Biol Clin Investig [Internet]. 28 janv 2017 [cité 18 juin 2025];31(2). Disponible sur: https://www.degruyter.com/document/doi/10.1515/hmbci-2017-0016/html
- Tian M, Zhou Y, Guo Y, Xia Q, Wang Z, Zheng X, et al. MicroRNAs in adipose tissue fibrosis: Mechanisms and therapeutic potential. Genes Dis. juill 2025;12(4):101287.
- Nguyen HP, An K, Ito Y, Kharbikar BN, Sheng R, Paredes B, et al. Implantation of engineered adipocytes suppresses tumor progression in cancer models. Nat Biotechnol [Internet]. 4 févr 2025 [cité 4 avr 2025]; Disponible sur: https://www.nature.com/articles/s41587-024-02551-2
- Slaughter VL, Rumsey JW, Boone R, Malik D, Cai Y, Sriram NN, et al. Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical therapeutic efficacy evaluation. Sci Rep. 23 juin 2021;11(1):13159.
- Huff LK, Amurgis CM, Kokai LE, Abbott RD. Optimization and validation of a fat-on-a-chip model for non-invasive therapeutic drug discovery. Front Bioeng Biotechnol. 25 juin 2024;12:1404327.
- McCarthy M, Brown T, Alarcon A, Williams C, Wu X, Abbott RD, et al. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng Part B Rev. 1 déc 2020;26(6):586‑95.
- Rebeaud M, Bouche C, Dauvillier S, Attané C, Arellano C, Vaysse C, et al. A novel 3D culture model for human primary mammary adipocytes to study their metabolic crosstalk with breast cancer in lean and obese conditions. Sci Rep. 22 mars 2023;13(1):4707.
FAQ
Adipose tissue is an endocrine organ that is central to metabolic health. It is not just a fat storage site. Its function is to store excess energy as triglycerides. This energy is released as fatty acids during periods of fasting. The tissue also secretes hormones, such as leptin and adiponectin. These hormones regulate appetite, insulin sensitivity, and inflammation. Adipose tissue also adapts its function in response to environmental stimuli like diet and stress. When this system is dysregulated, it contributes directly to metabolic diseases. This makes it a target for drug discovery.
Two main types of adipose tissue are recognised: white (WAT) and brown (BAT). WAT’s primary function is to store energy. In contrast, BAT burns energy. It generates heat through a process called non-shivering thermogenesis. WAT is further divided based on its location. Subcutaneous adipose tissue (SAT) is found beneath the skin. Visceral adipose tissue (VAT) is located around the abdominal organs. SAT is generally considered protective. It acts as a buffer against ectopic fat accumulation. VAT is more metabolically active. It is closely linked to insulin resistance and cardiovascular risk. Depot-specific differences in inflammation and lipolysis shape their distinct roles.
White adipose tissue (WAT) is the body’s principal caloric reservoir. It captures excess plasma glucose and free fatty acids. These are re-esterified into triglycerides. This process is controlled by lipogenic enzymes and the sympathetic nervous system. During fasting or adrenergic stress, these stores are mobilized by adipose-triglyceride-lipase and hormone-sensitive-lipase. This action fuels peripheral tissues. WAT is also an endocrine organ. Its unilocular adipocytes and resident immune cells secrete adipokines. These include leptin and adiponectin. These secretions modulate appetite and insulin action. Obesity causes adipocyte hypertrophy and ECM stiffening. The secretome is shifted toward pro-inflammatory signals, precipitating systemic insulin resistance.
The dysfunction of adipose tissue is a central cause of metabolic disorders. It is not a passive consequence of obesity. Conditions like insulin resistance and type 2 diabetes are now seen as originating from intrinsic defects in adipose depots. A loss of adipose tissue expandability is one of the earliest dysfunctions. When progenitor cell capacity is limited, excess lipids are shunted into existing adipocytes. This causes the cells to hypertrophy. These hypertrophic cells become stressed. They promote inflammation, lipotoxicity, and insulin resistance. The endocrine function of these cells is also disrupted. Beneficial adipokines are reduced, while pro-inflammatory cytokines are released. This inflammatory shift becomes systemic, affecting the liver, muscle, and pancreas.
The body accommodates excess energy in two different ways. In healthy individuals, a process called hyperplasia occurs. This is the generation of new, small adipocytes. These new cells remain insulin-sensitive. Tissue flexibility and metabolic homeostasis are preserved by this process. An unhealthy expansion occurs when this progenitor cell capacity is limited. This limitation can be due to genetics, aging, or inflammation. Excess lipids are then forced into existing adipocytes. This causes the cells to enlarge, a process called hypertrophy. These hypertrophic cells become metabolically stressed. They release pro-inflammatory cytokines. This leads to systemic insulin resistance.
Depot location is very important for metabolic health. Subcutaneous adipose tissue (SAT) is generally seen as protective. It tends to expand through hyperplasia and has lower inflammation. In contrast, visceral adipose tissue (VAT) is more lipolytically active. It is also more prone to immune cell infiltration. Current evidence points to VAT-derived inflammatory cytokines as main causes of metabolic dysfunction. These cytokines include IL-6, IL-1β, and RBP4. They are able to reach the liver through the portal circulation. Once there, stress pathways like JNK and NF-κB are activated. Insulin signaling is impaired. This promotes hepatic insulin resistance. This chronic inflammatory signaling appears to be the main harmful action of visceral fat.
Adipose depots have a storage limit. When this limit is reached, lipid overflow occurs. This leads to ectopic fat deposition. This means fat is deposited in non-adipose tissues. Examples include the liver, muscle, and pancreas. The liver condition is known as non-alcoholic fatty liver disease. Ectopic fat in muscle causes insulin resistance. Deposition in the pancreas can impair insulin secretion. These organs are not equipped for safe triglyceride storage. The accumulation of lipid intermediates, such as diacylglycerols and ceramides, follows. Intracellular signaling and mitochondrial function are disrupted by these intermediates. This phenomenon, known as lipotoxicity, is a trigger of organ dysfunction.
Hypoxia and fibrosis are pathological features of dysfunctional adipose tissue. As adipocytes enlarge (hypertrophy), their oxygen demand increases. The local vasculature fails to keep pace with this demand. The resulting lack of oxygen, or hypoxia, stabilizes hypoxia-inducible factor-1 alpha (HIF-1α). This factor promotes fibrotic remodeling instead of new blood vessel formation. An excess of extracellular matrix (ECM) is created. This matrix, composed of collagens and fibronectin, creates a stiffened environment. Lipid mobilization and nutrient exchange are impeded by this stiffness. Fibrosis also traps immune cells, which exacerbates local inflammation and worsens insulin resistance. This cycle of hypertrophy, hypoxia, and fibrosis is self-reinforcing.
Therapeutic strategies are increasingly focused on restoring healthy fat function. This is a shift from simply reducing fat mass. One approach is improving adipose tissue expandability. Drugs like thiazolidinediones (TZDs) activate PPAR-γ. This promotes hyperplasia, the formation of new, small adipocytes. Incretin-based therapies, such as GLP-1 receptor agonists, are also effective. They reduce appetite and visceral fat. Another strategy is targeting thermogenesis in brown and beige fat. Compounds like FGF21 analogues or β3-adrenergic agonists are studied to increase energy expenditure. Anti-fibrotic and immunomodulatory approaches are also being investigated. These aim to reverse tissue stiffening and chronic inflammation.
Our understanding of adipose tissue has relied heavily on animal models. Mouse models have been used to uncover mechanisms related to leptin and insulin resistance. Mice differ from humans in adipose distribution, thermogenic activity, and immune landscape. These differences limit the translation of findings into human therapies. In vitro, preadipocyte cell lines like 3T3-L1 have been used. These models are reproducible but lack cellular complexity and 3D architecture. They often fail to capture depot-specific differences, such as between visceral and subcutaneous fat. The field is now moving toward 3D culture systems. Adipose tissue organoids are derived from primary stromal vascular fractions or iPSCs. These 3D models offer a more faithful representation of human adipose physiology.