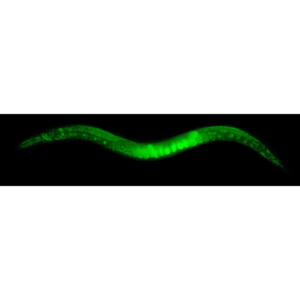
A new microfluidic system allows the high throughput phenotyping of C. elegans embryos!
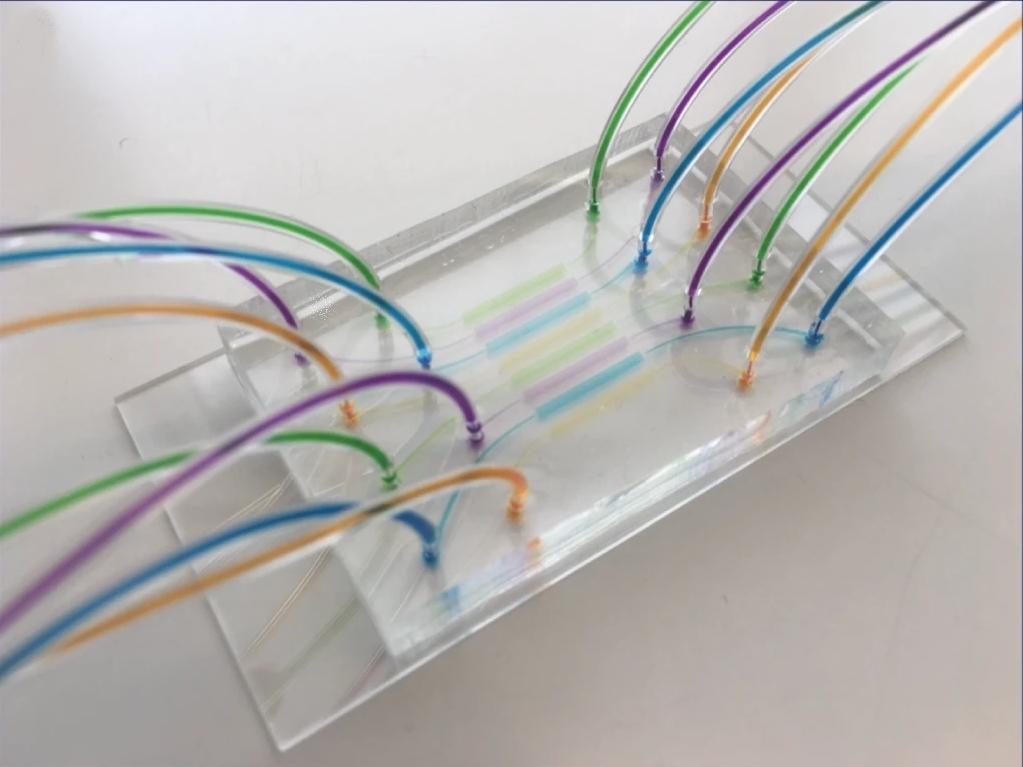
Martin A. M. Gijs et al. produced a high-throughput multiplexed microfluidic platform for automated phenotyping of C. elegans embryos during their full development, in order to make the culture faster and more efficient. Including deep learning and image processing, it produces accurate results in a relatively short amount of time. Each microfluidic lane can create different conditions to simultaneously observe the influence of various compounds on embryonic development.
As proof of concept, they made experiences with NaCl and glucose at different concentrations. Results show that the new microfluidic system has been able to analyze developmental lag and lethality due to molarity conditions. After this work, researchers suggest that C. elegans embryos may represent an alternative to adult worms as it leads to comparable experimental conclusions in a shorter time period.
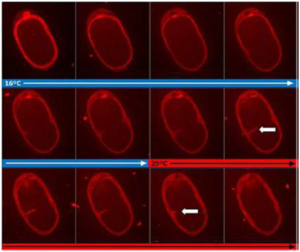
Ultra fast temperature shift device for in vitro experiments under microscopy
Abstract
The nematode Caenorhabditis elegans has been extensively used as a model multicellular organism to study the influence of osmotic stress conditions and the toxicity of chemical compounds on developmental and motility-associated phenotypes. However, the several-day culture of nematodes needed for such studies has caused researchers to explore alternatives. In particular, C. elegans embryos, due to their shorter developmental time and immobile nature, could be exploited for this purpose, although usually their harvesting and handling is tedious.
Here, we present a multiplexed, high-throughput and automated embryo phenotyping microfluidic approach to observe C. elegans embryogenesis after the application of different chemical compounds. After performing experiments with up to 800 embryos per chip and up to 12 h of time-lapsed imaging per embryo, the individual phenotypic developmental data were collected and analyzed through machine learning and image processing approaches. Our proof-of-concept platform indicates developmental lag and the induction of mitochondrial stress in embryos exposed to high doses (200 mM) of glucose and NaCl, while small doses of sucrose and glucose were shown to accelerate development. Overall, our new technique has potential for use in large-scale developmental biology studies and opens new avenues for very rapid high-throughput and high-content screening using C. elegans embryos.
References
- Automated phenotyping of Caenorhabditis elegans embryos with a high-throughput-screening microfluidic platform (Martin A. M. Gijs et al., 2020)
FAQ
A new microfluidic platform has been produced by researchers. It is designed for the automated analysis of C. elegans embryo phenotypes during their complete development. The system aims to make the culture process more efficient and faster. This platform is multiplexed, allowing for parallel experiments. This design means different conditions can be established in each microfluidic lane. The influence of various compounds on embryonic development can therefore be observed at the same time. A large number of embryos, up to 800, can be held on a single chip. Time-lapsed imaging for as long as 12 hours can be performed for every embryo. This new technique is proposed for large-scale developmental biology studies and for very rapid screening.
The nematode C. elegans has been widely used as a model multicellular organism. It is applied to studies on osmotic stress conditions and the toxicity of chemical compounds. These studies often focus on phenotypes associated with development and motility. A drawback is the culture time, which can be several days for adult nematodes. This has led researchers to explore other options. C. elegans embryos are one such alternative. They are useful due to their shorter developmental time. Their immobile nature is also a benefit. A new system was needed because the harvesting and handling of these embryos is typically tedious. It is suggested that embryos may represent a valid substitute for adult worms. Comparable experimental conclusions may be reached in a shorter time period.
The automated microfluidic platform is used to observe C. elegans embryogenesis. Observations are made after different chemical compounds have been applied. Experiments can be performed with as many as 800 embryos on each chip. Up to 12 hours of time-lapsed imaging can be recorded for each embryo. Following the imaging, individual phenotypic data on development is collected. This data is then analyzed. The analysis is performed using machine learning and image processing approaches. This combination of methods produces accurate results in a relatively short amount of time. This new technique is considered a way to conduct large-scale studies.
As a proof of concept, experiments were made using sodium chloride and glucose at different concentrations. The results demonstrated that the new microfluidic system was able to analyze developmental lag. Lethality due to the molarity conditions was also analyzed. The platform indicated that embryos exposed to high doses (200 mM) of glucose and NaCl experienced developmental lag. The induction of mitochondrial stress was also noted in these embryos. A different effect was seen with small doses. Small doses of sucrose and glucose were shown to accelerate the development of the embryos. These findings showed the system’s ability to measure the effects of different compounds.