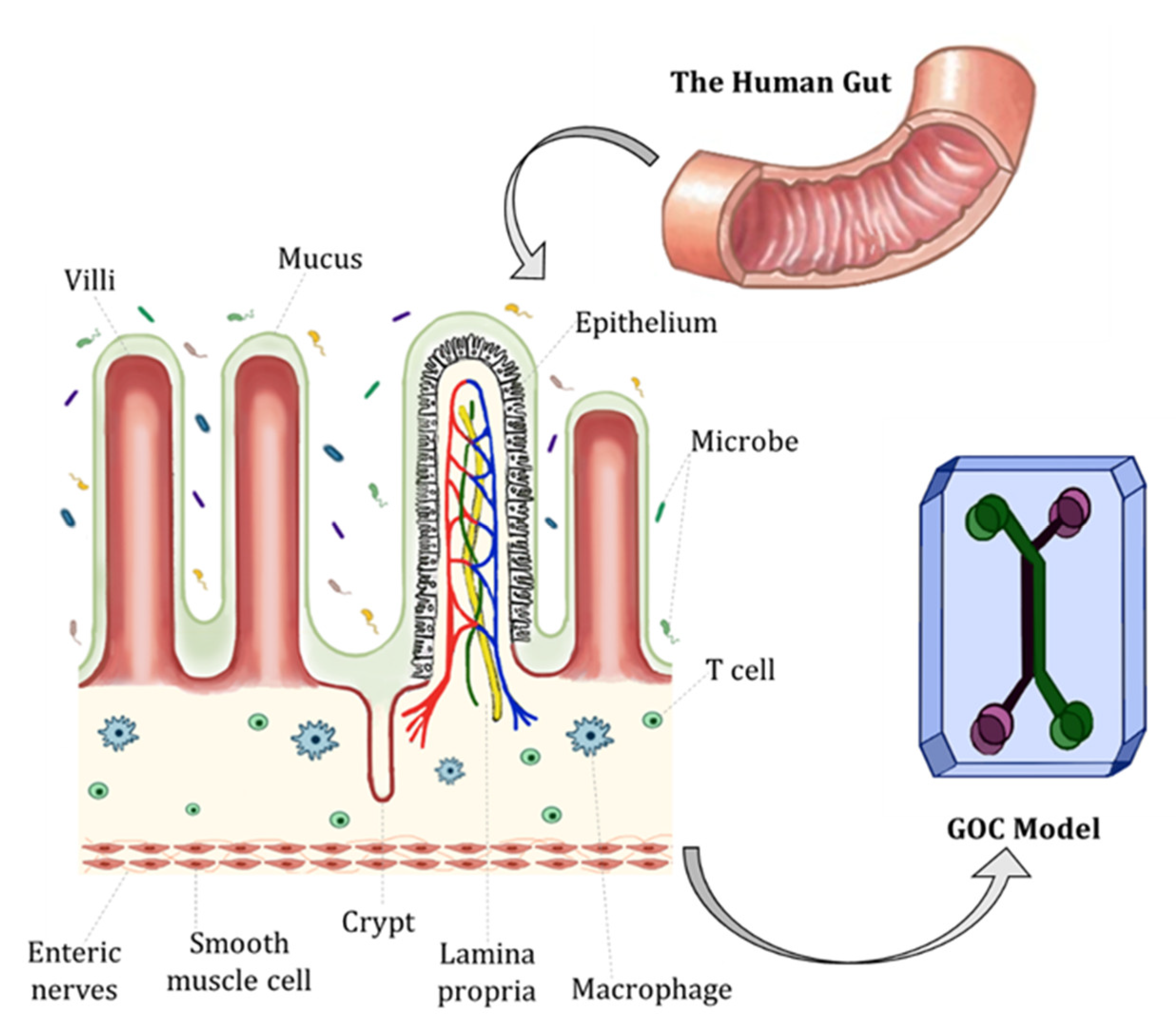
A complex human gut microbiome cultured in anaerobic intestine-on-a-chip
A recently published paper on Nature biomedical engineering highlights the possibility of cultivating gut microbiota on a gut-on-a-chip under aerobic and anaerobic condition. Interestingly, Jalili-Firoozinezhad S, Gazzinga F.S., Calamari E.L et colleagues demonstrated that the living gut microbiota can be stable in anaerobic condition for 5 days instead of 48 hours, cultivating the gut microbiota on chip with human Caco-2 intestinal epithelium or with cells cultivated from patients biopsies. This study represents a step forward on the production of organ-on-a-chip, and in particular for gut on chip design, gut-microbiota studies and characterizations, and personalized medicine approaches.
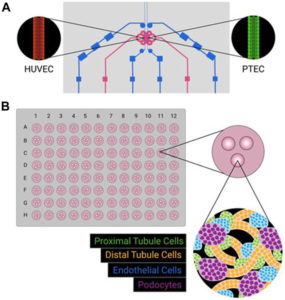
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Summary
The diverse bacterial populations that comprise the commensal microbiome of the human intestine play a central role in health and disease. A method that sustains complex microbial communities in direct contact with living human intestinal cells and their overlying mucus layer in vitro would thus enable the investigation of host-microbiome interactions. Here, we show the extended coculture of living human intestinal epithelium with stable communities of aerobic and anaerobic human gut microbiota, using a microfluidic intestine-on-a-chip that permits the control and real-time assessment of physiologically relevant oxygen gradients. When compared to aerobic coculture conditions, the establishment of a transluminal hypoxia gradient in the chip increased intestinal barrier function and sustained a physiologically relevant level of microbial diversity, consisting of over 200 unique operational taxonomic units from 11 different genera and an abundance of obligate anaerobic bacteria, with ratios of Firmicutes and Bacteroidetes similar to those observed in human faeces. The intestine-on-a-chip may serve as a discovery tool for the development of microbiome-related therapeutics, probiotics and nutraceuticals.
FAQ
The human intestine contains diverse bacterial populations. These populations form the commensal microbiome. This microbiome plays a central part in human health and disease. A method that can sustain these complex microbial communities in vitro is needed. Specifically, a system that allows them to be in direct contact with living human intestinal cells and their mucus layer would be beneficial. Such a method would permit the investigation of host-microbiome interactions. This research is important for understanding the microbiome’s role in the body.
A microfluidic intestine-on-a-chip has been developed. This system allows for the extended co-culture of living human intestinal epithelium with gut microbiota. It can sustain both aerobic and anaerobic microbes. A paper highlighted that living gut microbiota can be stable in anaerobic conditions for 5 days using this chip. This is an improvement from 48 hours. The chip can be used with human Caco-2 intestinal epithelium. It can also be used with cells cultivated from patient biopsies. This represents an advance in organ-on-a-chip design and gut-microbiota studies.
The microfluidic chip permits the control and real-time assessment of physiologically relevant oxygen gradients. A study compared aerobic co-culture conditions to the establishment of a transluminal hypoxia gradient. This hypoxia gradient was found to increase intestinal barrier function. It also sustained a physiologically relevant level of microbial diversity. This diverse community included over 200 unique operational taxonomic units from 11 different genera. An abundance of obligate anaerobic bacteria was supported. Furthermore, the ratios of Firmicutes and Bacteroidetes were similar to those observed in human faeces.
This intestine-on-a-chip technology is considered a step forward for personalized medicine approaches. It may also serve as a discovery tool. The system could be used for the development of microbiome-related therapeutics. Other potential applications include the development of probiotics. It could also be applied to the creation of nutraceuticals. The ability to sustain a complex microbiome in contact with human intestinal cells in vitro makes it a valuable platform. It will allow for new investigations into host-microbiome interactions.