In vitro liver models are central to drug discovery and toxicology, enabling controlled evaluation of metabolism, disposition, and safety before animal studies or clinical testing. In this first article, we present the main systems used across research and development including immortalized cell lines (e.g., HepG2, HepaRG) and primary hepatocytes, and we outline where each model typically fits in the workflow. Our goal is not to be exhaustive, but to provide a clear, practical map of the landscape so readers can align model choice with scientific questions and stage of development.
Because conventional two-dimensional culture imposes well-known constraints on function and longevity, we also introduce the rationale for moving toward three-dimensional (3D) formats as a way to enhance physiological relevance across models. A companion article builds on this foundation: there, we discuss the general advantages of 3D culture and, specifically for liver, the mechanistic reasons for improved predictivity: how restoring cell–cell and cell–matrix interactions, polarity, bile canaliculi, and transporter organization supports more durable and translational readouts.
Learn more about our ready to use human liver organoid models.
The main in vitro liver models and their applications
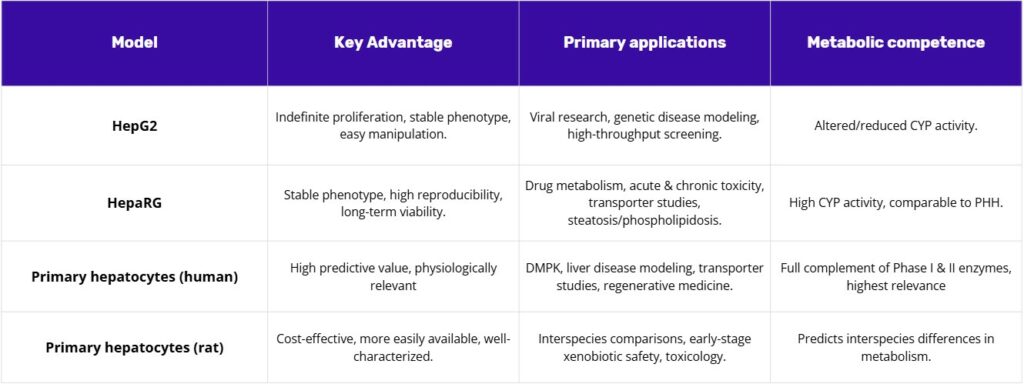
A range of in vitro liver models is available for preclinical research, each with distinct advantages, limitations, and use cases. The most widely used systems are immortalized cell lines such as HepG2 and HepaRG, alongside primary human and rat hepatocytes. Together, these models form a hierarchy of increasing physiological fidelity and complexity, allowing researchers to select the best tool for each stage of drug discovery and toxicology testing.
HepG2 Cells
HepG2 cells, an immortalized human hepatoma cell line, are a cornerstone of biological research, particularly for liver function and cancer studies. Originating from the liver tissue of a 15-year-old male with hepatocellular carcinoma, these cells are notable for their ability to proliferate indefinitely and maintain a stable, epithelial-like phenotype. Their robust nature and ease of manipulation make them a highly accessible and reproducible alternative to primary hepatocytes, which are limited by supply and donor variability. Despite their cancerous origin, HepG2 cells are non-tumorigenic and exhibit many differentiated hepatic functions, including the secretion of essential plasma proteins like albumin and plasminogen, which are crucial for protein-based assays(1).
Applications in Viral and Genetic Disease Modeling
HepG2 cells are indispensable for viral and genetic liver disease research. They serve as a critical tool for studying viral infections, demonstrating the complete cell cycle replication of hepatitis D (HDV) and the expression of hepatitis B (HBV) viral proteins. This susceptibility makes them a valuable platform for investigating viral life cycles, host-virus interactions, and for testing antiviral drugs, particularly in the context of HBV and HDV. Beyond infectious diseases, HepG2 cells are also employed to model various human liver diseases, from genetic conditions like progressive familial intrahepatic cholestasis (PFIC) and Dubin-Johnson Syndrome to chronic illnesses induced by exposure to cytotoxic and genotoxic agents(2).
Strengths in drug and toxin screening
The ease of use and reproducibility of HepG2 cells make them an optimal choice for high-throughput screening and stable cell line generation. Their compatibility with transfection studies, such as the use of luciferase reporter plasmids to track gene expression, is fundamental to metabolic research and drug targeting. Furthermore, the interaction of HepG2 cells with various biomaterials is pivotal in tissue engineering and the development of bio-artificial liver devices. Researchers study cell adhesion properties to determine viability for scaffolds and accurate liver tissue models, effectively using HepG2 as a versatile component in these advanced systems.
HepG2’s versatility stems from its consistent behavior across experiments, which is often a more critical factor in early-stage, large-scale screening than perfect physiological fidelity. While the low activity of certain xenobiotic metabolizing enzymes, particularly cytochrome P450s (CYPs), is frequently cited as a limitation, this characteristic can be strategically advantageous. By decoupling a compound’s toxicity from its metabolic activation, researchers can isolate and study direct cytotoxicity, which is a crucial first step in a thorough toxicological workflow. This approach highlights a fundamental principle in advanced modeling: the utility of a model is not solely determined by its resemblance to a native organ but by its ability to reliably address a specific scientific question. HepG2 cells serve as a prime example of a model that provides a stable, foundational platform for consistent, high-volume research.
HepaRG Cells
The HepaRG cell line represents a significant advancement in in vitro liver modeling, offering a solution that addresses the key shortcomings of traditional cell lines without incurring the logistical burdens of primary hepatocytes. Derived from a human hepatocellular carcinoma, HepaRG cells are unique in their ability to undergo a differentiation process that transforms them into a complex, organotypic co-culture of both hepatocyte-like and biliary-like cells. This spontaneous formation of “hepatocyte islands” surrounded by biliary-like epithelial cells closely mimics the native liver microarchitecture and provides a more physiologically relevant model even in a 2D format.
Superiority in metabolism and drug safety
The primary advantage of HepaRG cells is their high metabolic competence, which is markedly superior to that of HepG2 cells and closely approximates the levels seen in primary human hepatocytes. They exhibit high activity of Phase I and Phase II drug-metabolizing enzymes and full expression of key nuclear receptors (e.g., AhR, PXR, CAR), which are often lacking in other cell lines. This metabolic capability makes them a robust and reliable surrogate for primary cells in drug metabolism, disposition, and toxicity studies.
HepaRG cells are uniquely suited for predictive toxicology, as they can detect hepatotoxicants whose effects are dependent on metabolic activation or transporter function. For example, they show enhanced sensitivity to compounds like aflatoxin B1 and cyclophosphamide, which require biotransformation to exert their toxic effects, a capability that HepG2 cells lack. Additionally, HepaRG cells are the only known hepatic cell model to effectively predict specific toxicological endpoints such as steatosis and phospholipidosis(3).
Strengths in Drug-Drug Interaction and Transporter Studies
The high expression of drug transporters in HepaRG cells, which closely resembles that of primary human hepatocytes, is a critical advantage. Their ability to form tight junctions and functional bile canaliculi makes them an ideal model for studying transporter-mediated drug interactions, biliary secretion, and drug-induced cholestasis. Furthermore, HepaRG cells respond appropriately to prototypical CYP inducers such as omeprazole, phenobarbital, and rifampicin, making them a reliable tool for evaluating in vitro enzyme induction.
The HepaRG cell line occupies a unique position in the hierarchy of in vitro liver models, effectively bridging the gap between the simplicity of HepG2 and the physiological complexity of primary hepatocytes. It overcomes the metabolic and functional limitations of HepG2 while mitigating the major drawbacks of primary cells, namely donor variability, limited availability, and high cost. This makes them a “just right” model offering a stable, reproducible, and metabolically competent system for a wide array of drug discovery and toxicology applications. The spontaneous co-differentiation into hepatocyte-like and biliary-like cells is not merely a descriptive characteristic; it is a profound functional advantage that allows the model to capture crucial inter-cellular interactions necessary for proper liver function, which is essential for studying complex mechanisms of drug-induced liver injury (DILI).
Primary hepatocytes: The gold standard and benchmark
For decades, freshly isolated primary hepatocytes have been considered the pinnacle of in vitro liver modeling. Their high predictive value and comprehensive metabolic capacity make them the gold standard against which all other models are measured.
Primary Human Hepatocytes (PHH)
Primary human hepatocytes (PHH) possess the full, native complement of Phase I and II metabolic enzymes, nuclear receptors, and drug transporters at physiologically relevant levels. This makes them an unparalleled model for in vitro studies of drug metabolism and pharmacokinetics (DMPK), liver disease modeling, and toxicology. Their use provides the earliest and most accurate insights into how a drug will behave in the human body, helping to reduce the risk of adverse effects in clinical trials. PHH are also invaluable for transporter studies, regenerative medicine, and for the development of microphysiological systems (MPS)(4,5).
Primary Rat Hepatocytes (PRH)
Primary rat hepatocytes (PRH) offer a cost-effective and readily available alternative to their human counterparts. They are widely used for early-stage xenobiotic safety assessment and for preclinical studies requiring a high volume of cells. A particular strength of PRH is their utility in predicting interspecies differences in drug metabolism, which is a critical step in translating preclinical findings to human clinical trials. They provide a reliable system for characterizing the metabolism of test substances and can be used to assess toxicity and enzyme induction.
A central challenge with primary hepatocytes, both human and rat, lies in the inherent conflict between their high physiological relevance and their practical limitations. Their utility is significantly hampered by rapid dedifferentiation in standard 2D culture, leading to a loss of key drug-metabolizing activities within hours or a few days. Furthermore, their reliance on donor organs results in poor scalability, limited availability, and significant batch-to-batch variability, which can complicate data reproducibility. These technical, economic, and strategic constraints are the fundamental drivers for the development of alternative models, such as HepG2 and HepaRG, and for the advancement of 3D culture platforms that can better preserve their function over time. The fact that primary hepatocytes are now routinely integrated into advanced systems like MPS and 3D cultures demonstrates that researchers are not abandoning the gold standard but are actively working to overcome its limitations by placing it in more supportive, in vivo-like environments(6,7).
Conclusion
In summary, current in vitro liver systems offer a range of options that researchers can select based on their specific needs and project phases. However, these models share a common drawback: cells cultured in standard 2D conditions tend to lose functionality over time, making it difficult to study processes that unfold over extended periods. This has led researchers to increasingly explore 3D culture methods, which better mimic the liver’s natural environment and tend to yield more accurate predictions.
In the companion article, we examine how 3D culture systems enhance cell behavior; not by changing the cells themselves, but by providing a more supportive environment. This environmental improvement helps maintain cellular function over longer periods, allows for more comprehensive analysis, and ultimately produces data that more closely reflects actual human biology. The result is a clearer path from early testing to generating reliable evidence for decision-making.
References
- Arzumanian VA, Kiseleva OI, Poverennaya EV. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int J Mol Sci. 4 déc 2021;22(23):13135.
- Verrier E, Colpitts C, Schuster C, Zeisel M, Baumert T. Cell Culture Models for the Investigation of Hepatitis B and D Virus Infection. Viruses. 20 sept 2016;8(9):261.
- Andersson TB, Kanebratt KP, Kenna JG. The HepaRG cell line: a unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin Drug Metab Toxicol. juill 2012;8(7):909‑20.
- Heydari Z, Gramignoli R, Piryaei A, Zahmatkesh E, Pooyan P, Seydi H, et al. Standard Protocols for Characterising Primary and In Vitro‐Generated Human Hepatocytes. J Cell Mol Med. févr 2025;29(3):e70390.
- Vildhede A, Wiśniewski JR, Norén A, Karlgren M, Artursson P. Comparative Proteomic Analysis of Human Liver Tissue and Isolated Hepatocytes with a Focus on Proteins Determining Drug Exposure. J Proteome Res. 7 août 2015;14(8):3305‑14.
- Yun J, Jeon TJ, Kim SM. Current Advances and Future Perspectives of Liver-on-a-Chip Platforms Incorporating Dynamic Fluid Flow. Biomimetics. 4 juill 2025;10(7):443.
- Shao W, Xu H, Zeng K, Ye M, Pei R, Wang K. Advances in liver organoids: replicating hepatic complexity for toxicity assessment and disease modeling. Stem Cell Res Ther. 26 janv 2025;16(1):27.
FAQ
In vitro liver models are a central part of drug discovery and toxicology. Controlled evaluations of metabolism, disposition, and safety are enabled by these systems. This testing is performed before animal studies or clinical trials are conducted. A hierarchy of models is available, ranging in physiological fidelity and complexity. These systems include immortalized cell lines and primary hepatocytes. This range allows the selection of an appropriate tool for each stage of testing. The models form a foundation for preclinical liver research. Their predictive value can be constrained by the shortcomings of conventional two-dimensional culture, which has led to exploration of 3D formats.
HepG2 cells are an immortalized human hepatoma cell line. They are a common tool in biological research, especially for liver function and cancer studies. The line originated from the liver tissue of a 15-year-old male who had hepatocellular carcinoma. These cells are known for their ability to proliferate indefinitely. They also maintain a stable, epithelial-like phenotype. Their stable nature and ease of manipulation make them an accessible and reproducible option. They are non-tumorigenic. Many differentiated hepatic functions are exhibited, such as the secretion of plasma proteins like albumin.
Research into viral and genetic liver diseases relies on HepG2 cells. They are used to study viral infections. The complete cell cycle replication of hepatitis D (HDV) has been demonstrated in them. The expression of hepatitis B (HBV) viral proteins also occurs. This susceptibility makes them a useful platform for investigating viral life cycles. Host-virus interactions can be examined. Antiviral drugs, especially for HBV and HDV, are also tested using this model. Beyond infectious diseases, HepG2 cells are employed to model other human liver conditions. These include genetic conditions like progressive familial intrahepatic cholestasis.
Yes, this characteristic can be strategically employed. The low activity of certain xenobiotic metabolising enzymes, like cytochrome P450s (CYPs), is often cited as a shortcoming. This feature allows for the decoupling of a compound’s toxicity from its metabolic activation. Researchers can isolate and study direct cytotoxicity. This is an important first step in a thorough toxicological workflow. The utility of a model is determined by its ability to reliably address a specific scientific question. It is not solely based on its resemblance to a native organ. HepG2 cells offer a stable, foundational platform for consistent, high-volume research.
The HepaRG cell line is derived from a human hepatocellular carcinoma. These cells are distinct because of their ability to undergo a differentiation process. This process transforms them into a complex, organotypic co-culture. The co-culture contains both hepatocyte-like and biliary-like cells. This spontaneous formation results in “hepatocyte islands” that are surrounded by biliary-like epithelial cells. This arrangement closely mimics the native liver microarchitecture. A more physiologically relevant model is presented, even when used in a 2D format. They offer a system that addresses shortcomings of other cell lines without the logistical burdens of primary cells.
The principal benefit of HepaRG cells is their high metabolic competence. This competence is markedly greater than that of HepG2 cells. It also closely approximates the levels seen in primary human hepatocytes. High activity of Phase I and Phase II drug-metabolising enzymes is exhibited. Full expression of certain nuclear receptors (such as AhR, PXR, CAR) is also observed. These receptors are often lacking in other cell lines. This metabolic capability makes HepaRG cells a stable and reliable surrogate for primary cells. They are well-suited for drug metabolism, disposition, and toxicity studies.
HepaRG cells are uniquely suited for predictive toxicology. They can detect hepatotoxicants whose effects are dependent on metabolic activation or transporter function. An enhanced sensitivity is shown to compounds like aflatoxin B1. These compounds require biotransformation to exert their toxic effects. This is a capability that HepG2 cells lack. In addition, HepaRG cells are the only known hepatic cell model to effectively predict specific toxicological endpoints. These endpoints include steatosis and phospholipidosis. Their high expression of drug transporters, which resembles primary human hepatocytes, is another benefit. This makes them a good model for studying drug-induced cholestasis.
Primary human hepatocytes possess the full, native complement of metabolic enzymes. This includes Phase I and Phase II enzymes. They also have nuclear receptors and drug transporters at physiologically relevant levels. This makes them an unparalleled model for in vitro studies. They are used in drug metabolism and pharmacokinetics (DMPK), liver disease modeling, and toxicology. Their use gives the earliest and most accurate information on how a drug might behave in the human body. This information helps to reduce the risk of adverse effects in clinical trials. They are also used for transporter studies and in the creation of microphysiological systems.
Primary rat hepatocytes (PRH) are a cost-effective and readily available alternative to human cells. They are commonly used for early-stage xenobiotic safety assessment. They are also used for preclinical studies that require a high volume of cells. A particular use for PRH is in predicting interspecies differences in drug metabolism. This is an important step for translating preclinical findings to human clinical trials. A reliable system is offered by PRH for characterising the metabolism of test substances. Toxicity and enzyme induction can also be assessed with this model.
A central challenge exists with primary hepatocytes, both human and rat. Their utility is hampered by rapid dedifferentiation in standard 2D culture. This leads to a loss of their drug-metabolising activities within hours or a few days. Their reliance on donor organs creates further problems. This reliance results in poor scalability and limited availability. Significant batch-to-batch variability is also introduced, which can complicate data reproducibility. These technical, economic, and strategic constraints are the principal drivers for the creation of alternative models. These drawbacks are also the reason for the advancement of 3D culture platforms, which can better preserve their function.