Intestine: Overview, Histology and Pathology
The intestine is the largest part of the alimentary canal, which is part of the gastrointestinal tract (also called ‘gut’) that, with accessory glands, form the digestive system. The intestine is divided into small intestine (duodenum, jejunum, and ileum) and large intestine (cecum, colon, rectus, and anus). The small intestine is where most of the digestion and absorption occurs; the large intestine completes the absorption of nutrients and water, synthesise certain vitamins, produce faeces, and excrete the substances eliminated from the body. Intestinal Biopsies
The intestinal histology, including the histology of small and large intestine, is described in figure 1.
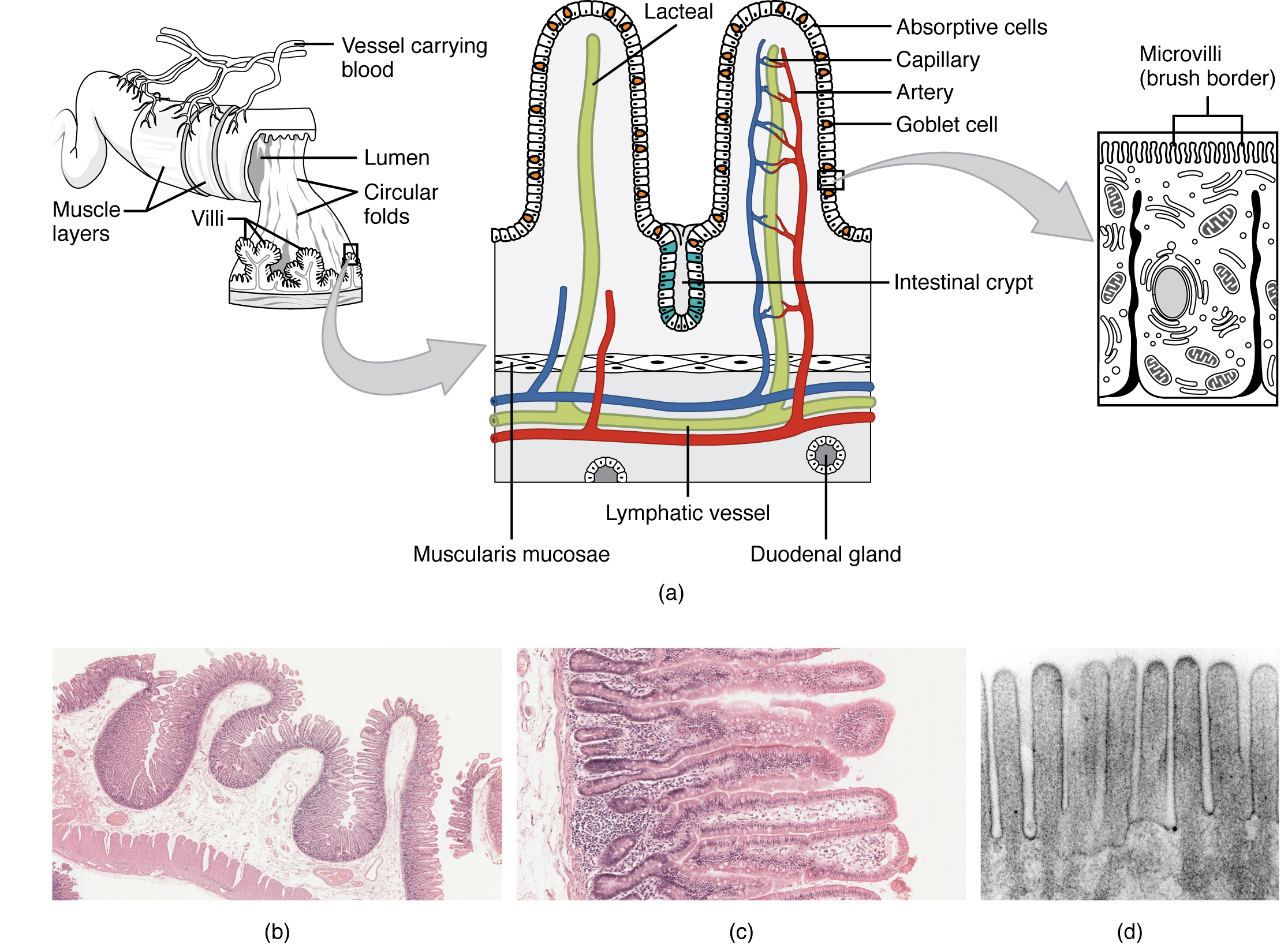
Figure 1: Schematic representation of the anatomy and the histology of intestine. (a) Tissue layers of the gut: mucosa, submucosa, muscularis, and serosa. (b) Histology of small intestine represented by villi, microvilli and circular folds with the function of increase the absorptive surface of the intestine. (c) Histology of large intestine mostly characterised by columnar epithelium (enterocytes and goblet cells) and no circular folds or villi; enterocytes absorb water, salts, vitamins and substances produced by the gut microbiota and the goblets cells produce mucus to protect the intestine from toxins and gases released by the bacteria and to facilitate the evacuation. The figure is adapted by CC 3.0 BY OpenStax College, ‘Anatomy & Physiology’, Connexions Web site http://cnx.org/content/col11496/1.6/, Jun 19, 2013
The intestinal epithelium is characterised by proliferative crypts where stem cells resides, and cells with absorptive and secretory functions (enterocytes, goblet cells, endocrine cells, Paneth cells, M cells, and tuft cells). The enteric nervous system, mesenchymal and immune cells contributes to maintain and regulate the intestinal morphology and physiology [1].
A wide range of disease affect the intestinal epithelium as such inflammatory bowel disease (IBD), celiac disease, infectious diseases, diarrheas, and intestinal cancers. Interestingly, in the recent years the interactions between host and intestinal microbiota in physiology and pathology is acquiring the attentions of research in the medical fields. The intestinal lumen gas composition is still debated and is strongly influenced by the gut microbiota; it is suggested that the gas composition can be associated to intestinal dysbiosis and pathologies [2–4].
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Intestinal Biopsies – Clinical evidence and diagnostic parameters
Nowadays, remarkable revolutions in medical imaging are occurring, impacting in the cost of screening, diagnosis and health care. Notwithstanding this technology’s advances, in the bowel diseases, endoscopy combined with histopathology analysis are the best methods to detect abnormalities and provide precise diagnosis. The biopsy of the small intestine is not very common and is relevant for the diagnosis of Whipple’s disease and celiac disease[5], [6]. Polyps at the duodenal level are usually benign lesions and the cancers in this part of the intestine are just the 5% of the gastrointestinal tumours. Histologically malignant small bowel tumours are adenocarcinomas, endocrine tumours, lymphomas and soft tissue tumours. Tissue histopathology may be collected during the cholangiopancreatography (ERCP) by brushing, biopsy, and bile aspiration [7].
In the terminal ileum and colon, biopsy is useful in several conditions:
- Diarrhoea and colitis[8], [9]
- Amyloidosis and rare metabolic lysosomal or storage disorders[10]
- IBD: ulcerative colitis and Chron’s disease (differential diagnosis, grading and follow-up) [11]
- Pouchitis
- Eosinophilic disorders
- Dysplasia and
- Cancer [12]
Gastrointestinal Neuromuscular diseases is necessary a full thickness intestinal biopsy obtained by surgery [13].
Intestinal Biopsies and Precision-cut Intestinal Slices – Preclinical and Clinical towards Personalised medicine and Disease Investigation
The gut plays a vital role in the absorption of nutrients and drugs, and elimination of endogenous and exogenous compounds. The intestine express also numerous drug metabolic enzymes (DME) and drug transporters (DT), showing a primary role in the first-pass metabolism that before was attributed just to the liver [14]. Thus, the increased use of biopsy and precision-cut intestinal slices have shown to have high potential as in vitro model for drug metabolism, drug interaction and transport. In addition, intestinal and liver culture can be studied in perfused biochip system [15]. Moreover, in vitroand ex vivomethodologies has been explored to identify models able to represent the complex anatomy and physiology of the intestine.
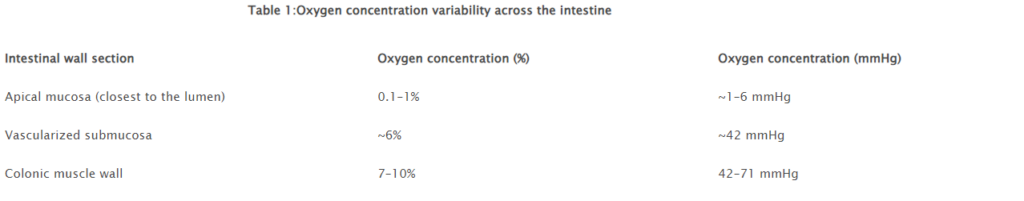
The increased use of biopsy and precision-cut intestinal slices have shown to have high potential as in vitro model for drug metabolism, drug interaction and transport. Maintaining ex vivocultures of human intestines is challenging because of: (i) the heterogeneous tissue (epithelial, immune and neuronal cells); (ii) and the different oxygen gradient concentration [16] (Table 1). Alterations of oxygen in the gut wall are strongly linked with the gut microbiota both in vivo[17]and in vitro[18]and, therefore, also influenced by the antibiotic treatments. Intestinal Biopsies
The survival rate of the cells in slices is up 72h: the mean rate of died cells is of 60 +/- 11.9 cell/mm2across 24h and 48h ex vivo; at 96h the tissue viability decreases and the degradation of patterned rows of crypts is observed. Recapitulating oxygen gradient ex vivo and prolonging the survival tissue rate is challenging, but fundamental for better study the physiology and pharmacology response of intestine. Optimised model and further investigation are necessary for translational potential, including enteric pathogen interactions and hot-microbiome interaction.
References
[1] C. A. Richmond and D. T. Breault, “Move Over Caco-2 Cells: Human-Induced Organoids Meet Gut-on-a-Chip,” Cmgh, vol. 5, no. 4, pp. 634–635, 2018.
[2] J. Z. Ou, C. K. Yao, A. Rotbart, J. G. Muir, P. R. Gibson, and K. Kalantar-zadeh, “Human intestinal gas measurement systems: In vitro fermentation and gas capsules,” Trends Biotechnol., vol. 33, no. 4, pp. 208–213, 2015.
[3] M. D. Levitt and J. H. Bond, “Volume, composition, and source of intestinal gas.,” Gastroenterology, vol. 59, no. 6, pp. 921–929, 1970
[4] K. Kalantar-Zadeh et al., “Intestinal Gas Capsules: A Proof-of-Concept Demonstration,” Gastroenterology, vol. 150, no. 1, pp. 37–39, 2016.
[5] S. Husby et al., “European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease,” J. Pediatr. Gastroenterol. Nutr., vol. 54, no. 1, pp. 136–160, 2012.
[6] A. S. Mee, M. Burke, A. G. Vallon, J. Newman, and P. B. Cotton, “Small bowel biopsy for malabsorption: Comparison of the diagnostic adequacy of endoscopic forceps and capsule biopsy specimens,” Br. Med. J. (Clin. Res. Ed)., vol. 291, no. 6498, pp. 769–772, 1985.
[7] M. Torabi et al., “We are IntechOpen , the world ’ s leading publisher of Open Access books Built by scientists , for scientists TOP 1 %,” Intech, vol. i, no. tourism, p. 13, 2016.
[8] A. Prior, A. M. Lessells, and P. J. Whorwell, “Is biopsy necessary if colonoscopy is normal?,” Dig. Dis. Sci., vol. 32, no. 7, pp. 673–676, 1987.
[9] R. J. Shah, C. Fenoglio-Preiser, B. L. Bleau, and R. A. Giannella, “Usefulness of colonoscopy with biopsy in the evaluation of patients with chronic diarrhea,” Am. J. Gastroenterol., vol. 96, no. 4, pp. 1091–1095, 2001.
[10] R. Kirsch et al., “Systemic mastocytosis involving the gastrointestinal tract: Clinicopathologic and molecular study of five cases,” Mod. Pathol., vol. 21, no. 12, pp. 1508–1516, Dec. 2008.
[11] R. M. Feakins, “Inflammatory bowel disease biopsies: Updated British Society of Gastroenterology reporting guidelines,” J. Clin. Pathol., vol. 66, no. 12, pp. 1005–1026, 2013.
[12] F. D. M. Van Schaik et al., “Role of immunohistochemical markers in predicting progression of dysplasia to advanced neoplasia in patients with ulcerative colitis,” Inflamm. Bowel Dis., vol. 18, no. 3, pp. 480–488, 2012.
[13] G. Hajiyeva and S. Ngamruengphong, “Endoscopic techniques for full thickness intestinal biopsy,” Curr. Opin. Gastroenterol., vol. 34, no. 5, pp. 295–300, 2018.
[14] I. de Waziers, P. H. Cugnenc, C. S. Yang, J. P. Leroux, and P. H. Beaune, “Cytochrome P 450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues.,” J. Pharmacol. Exp. Ther., vol. 253, no. 1, 1990.
[15] P. M. Van Midwoud, M. T. Merema, E. Verpoorte, and G. M. M. Groothuis, “A microfluidic approach for in vitro assessment of interorgan interactions in drug metabolism using intestinal and liver slices,” Lab Chip, vol. 10, no. 20, pp. 2778–2786, 2010.
[16] L. A. Schwerdtfeger, N. J. Nealon, E. P. Ryan, and S. A. Tobet, “Human colon function ex vivo: Dependence on oxygen and sensitivity to antibiotic,” PLoS One, vol. 14, no. 5, pp. 1–17, 2019.
[17] L. Albenberg et al., “Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota,” Gastroenterology, vol. 147, no. 5, pp. 1055-1063.e8, 2014.
[18] J. Z. H. von Martels et al., “The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut,” Anaerobe, vol. 44, pp. 3–12, 2017
FAQ
The intestine is the largest portion of the alimentary canal. It is divided into the small intestine and the large intestine. Most digestion and absorption of nutrients occurs in the small intestine. The large intestine’s functions include completing nutrient and water absorption, synthesizing certain vitamins, and producing faeces for excretion. The intestine has several tissue layers, including the mucosa, submucosa, muscularis, and serosa. The small intestine’s histology is noted for villi and microvilli, which increase its absorptive surface. The large intestine lacks villi and is mostly characterized by columnar epithelium, including enterocytes for absorption and goblet cells that produce mucus.
Despite advancements in medical imaging, endoscopy combined with histopathology analysis remains the best method for detecting abnormalities in bowel diseases. Biopsies of the small intestine are not very common. They are considered relevant for the diagnosis of specific conditions like Whipple’s disease and celiac disease. In the terminal ileum and colon, biopsies are useful for a wider range of conditions. These include diarrhoea and colitis, amyloidosis, and inflammatory bowel diseases such as ulcerative colitis and Chron’s disease. They are also used for diagnosing dysplasia and cancer. For gastrointestinal neuromuscular diseases, a full-thickness intestinal biopsy obtained through surgery is necessary.
The intestine is involved in the absorption of nutrients and drugs. It also eliminates endogenous and exogenous compounds. The intestine is now known to have a primary part in first-pass metabolism, which was previously attributed just to the liver. This is because the intestine expresses numerous drug metabolic enzymes and drug transporters. The increased use of biopsy tissue and precision-cut intestinal slices has shown these are promising in vitro models. They are applied to the study of drug metabolism, drug interaction, and transport. Intestinal and liver cultures can also be studied together within a perfused biochip system.
Maintaining ex vivo cultures of human intestinal tissue is known to be difficult. This is due to several factors. One reason is the heterogeneous nature of the tissue, which includes epithelial, immune, and neuronal cells. Another issue is the presence of different oxygen gradient concentrations within the tissue. Alterations of oxygen in the gut wall are closely linked with the gut microbiota. The survival rate for cells in these slices is limited, lasting up to 72 hours. By 96 hours, tissue viability decreases, and the degradation of crypts is observed. Recapitulating the correct oxygen gradient ex vivo and prolonging the tissue’s survival rate is a requirement for better pharmacological studies.
FAQ
The liver is the human body’s largest organ. It is responsible for 1st and 2nd pass metabolism. The organ is a center for nutritional and drug metabolism. It also has an important part in the excretory system, removing endogenous waste or xenobiological compounds. Optimal operation requires stable temperature (normothermia) and oxygen supply (normoxia). The tissue contains hepatocytes, Kupffer cells, stellate cells, and others. Liver models are considered necessary for pharmacological applications. These applications relate to absorption, distribution, metabolism, excretion, and toxicology (ADMET). Mechanistic studies of how pathologies begin and advance are also conducted.
Biomarkers for liver events are often found in blood and urine, but a liver biopsy provides a definitive diagnosis. The procedure can also assist in prognosis. Biopsies are done using percutaneous, laparoscopic, or transvenous methods. Guidance is typically provided by ultrasound, MRI, or CT imaging. Biopsies are required for several types of liver disease. Examples include drug-induced liver injury or sensitivity to chemotherapy. They are also used for non-alcoholic steatohepatitis (NASH), cirrhosis, viral infections, and hepatic cancer. Additionally, biopsies are used to determine the viability of liver tissue for a transplant and for post-transplant analysis.
Precision-cut slices, or PCS, of liver biopsy tissue permit the study of liver tissues in an ex vivo setting. This method preserves the tissue’s complexity. This includes the original cell types and ratios. An intact, native extracellular matrix is also maintained. Adopting PCS methods offers benefits compared to 2D monocultures. In 2D culture, hepatocytes lose many phenotypic properties within 24 to 48 hours, especially the expression of cytochrome p450 enzymes. Hepatocytes in 2D are also hypersensitized to chemotherapeutic compounds. Furthermore, 2D cultures lack immune system interactions and other cell-to-cell communication. They also do not have biomechanical stimulation or interaction with an extracellular matrix.
Static liquid culturing of liver biopsy tissue, which is dependent on an incubator, has several difficulties. These are shared with many other in vitro methods. A loss of cellular phenotypes and overall tissue integrity can occur within 24 to 72 hours. Tissue necrosis is another problem. This is attributed to hypoxia from poor oxygen diffusion in the liquid culture. Non-physiological accumulation of waste also occurs in static media. Additionally, temperature shocks (hypothermia) happen when tissues are moved from a 37°C incubator to a biocabinet. While bioreactor systems with perfusion address most of these issues, current commercial systems are expensive. Accessible technology is needed to provide normoxia, normothermia, and physiological nutrient supply.