Introduction
Two-photon excitation microscopy is a particularly microscopy technique based on the capability, under specific circumstances, to excite with two photons one electron in the ground state. The particularity of this phenomenon concern the fact that the light used to induce the excitation has a lower wavelength than the collected fluorescent. This makes two-photon microscopy particularly cell-friendly and allows among others, to image living specimens with little to no physiological alterations.
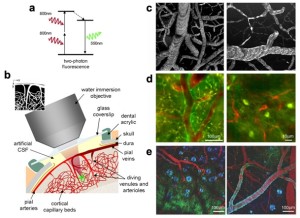
Legend : Two-photon microscopy of in vivo brain function. (a) Basic mechanism of two-photon fluorescence. (b) Schematic of surgical preparation of exposed cortex, with sealed glass window and microscope objective positioning. Green dot shows location of two-photon fluorescence. (c) Examples of two-photon maps of the vasculature following intravenous injection of dextran-conjugated fluorescein. Black dots and stripes show red blood cell motion. (d) Dual-channel imaging of neuronal (green) and vascular (red) signals: (left) Oregon Green 488 BAPTA-1 AM calcium sensitive dye stained neurons and (right) transgenic mouse expressing green fluorescent protein (GFP) in a subpopulation of neurons (mouse supplied by Jeffrey M. Friedman, Rockefeller University, New York) [101]. Texas dextran red is the intravascular tracer in both cases. (e) Three channel imaging of Tg2576 APP Alzheimer’s disease mouse model with amyloid-targeting dye (blue), GFP expressing neurons and dendrites (green) and vasculature (red). Source https://commons.wikimedia.org/wiki/File:Two-photon_microscopy_of_in_vivo_brain_function.jpg adapted from [1] and contributed by Elizabeth Hillman (Columbia University, New York).
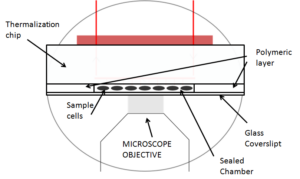
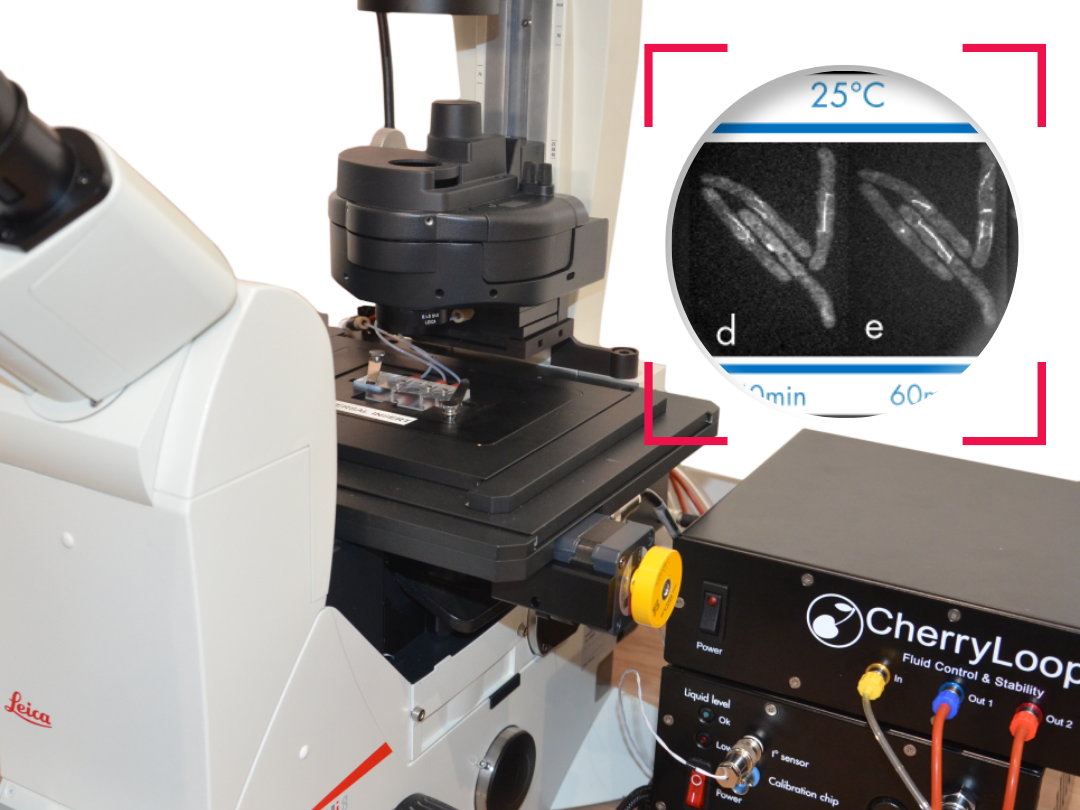
Ultra fast temperature shift device for in vitro experiments under microscopy
Principle of two-photon microscopy
Two-photon microscopy is based on the principle originally predicted by M. Goeppert-Mayer in 1931 and then demonstrated by W. Kaiser and C.G.B Garrett in 1961. The phenomenon concerns the absorption of two photons of identical or different wavelengths in order to excite a molecule. Most of fluorescence microscopy techniques are based on single photon absorption (figure 2). In single photon absorption the electron of a fluorophore is excited by a wavelength that has more energy than the one emitted by the fluorescence phenomenon. Traditionally UV excitation is used to collect blue fluorescence, blue light to excite fluorophores that emit in the green and so on. In two photon microscopy the electron of the fluorophore is excited by two photons with a longer wavelength than the resulting fluorescence emission (Figure 2). Contrary to single absorption, two-photon absorption is more difficult to obtain at lower light intensity since it is a second-order process. On the other hand when photons are dense enough two-photon absorption prevails over the linear single absorption.
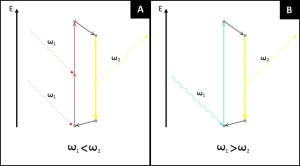
Legend : (A) In single photon absorption an electron hit by a photon with sufficient energy to excite it jumps to an higher energetic level. Through internal conversions the excited electron lose part of its energy (non-radiative transition, black arrow) before eventually emit fluorescence. The energy of the exciting photons is always bigger than the emitted one. (B) In two photon absorption an electron is moved from the ground level by a first photon, to reach the excited state the photon must absorb a second photon while the electron is in its virtual state. The energy of the two photons is completely absorbed by the electron (normally in its ground state) and the emitted fluorescence has a shorter wavelength than the exciting light.
Two-photon microscopes: main advantages
The optical scheme of a two photon microscope is not much different from a standard laser scanner confocal microscope. In both technologies a spotted light is produced by a laser source passing through a pinhole. Two galvanic mirrors then manipulate the angle of the light in order to scan the sample. The main differences concern the lack of the sample pinhole and the illumination source.
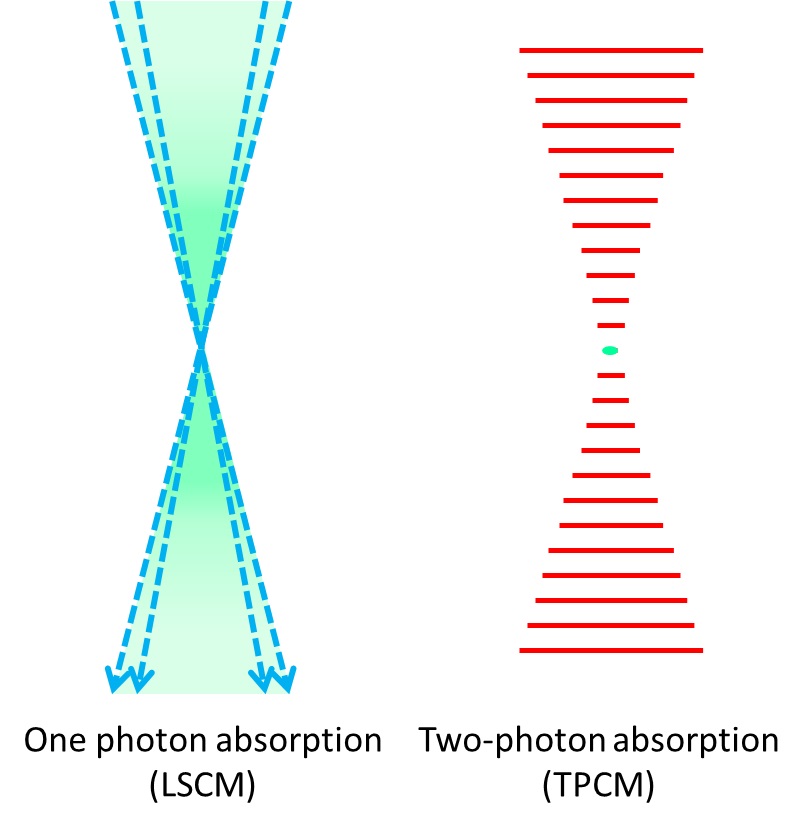
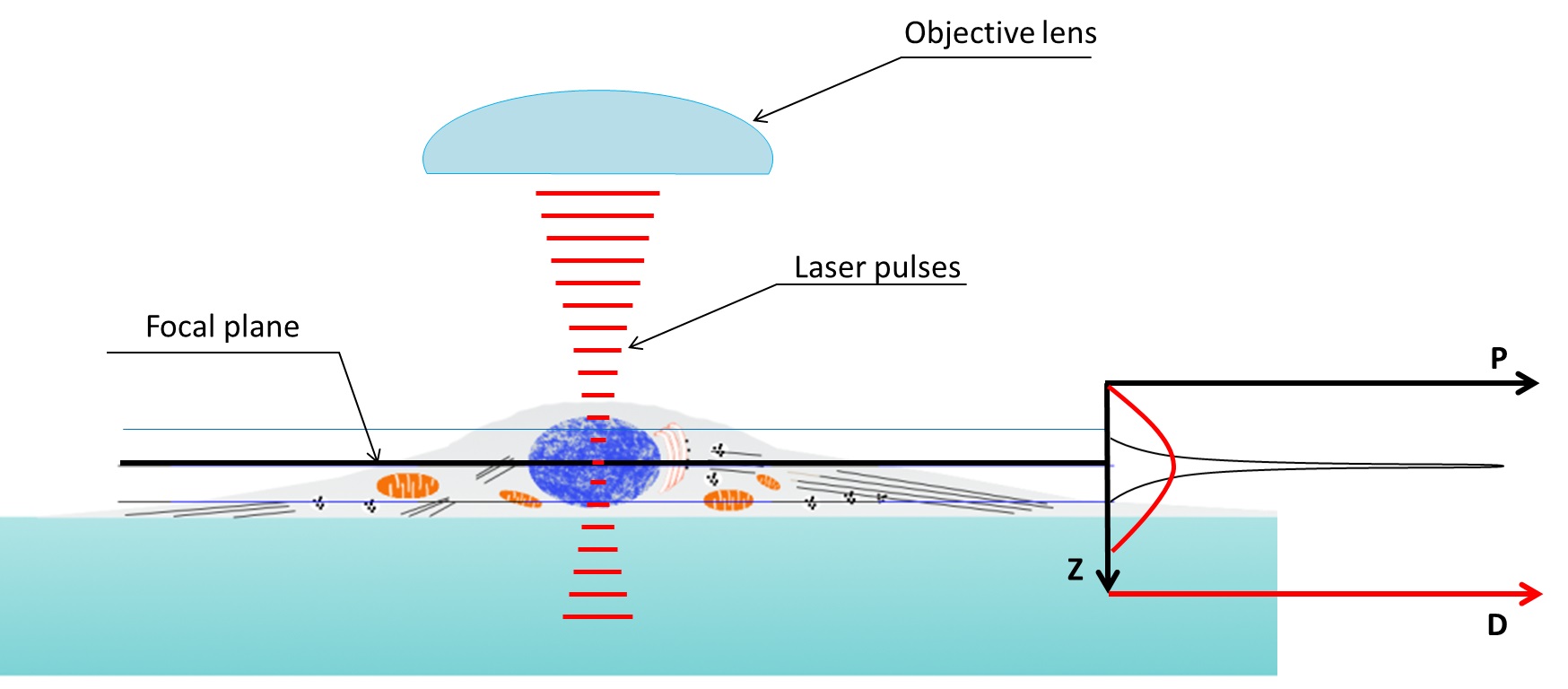
In confocal microscopy sample pinhole is in charge of filtering the fluorescence that originates at the focal plane. In two-photon microscopy this is not needed thanks to the different phenomenon used to excite fluorophores (figure 3). The physical principle of two-photon absorption dictates that only at high photon density the phenomenon prevails over one photon absorption. The relation is a second order relation and implies that only when photons are concentrated enough fluorescence occurs (figure 4). This brings to two photon microscopy a better resolution in the z axis when compared to confocal microscopes.
The second difference is related to the laser. While confocal microscopes laser emission is rather constant, two-photon microscopy uses pulsed laser in order to concentrate as much photons as possible in each single pulse.
The way the microscope illuminates the sample in a two-photon microscopy brings several advantages when compared to confocal microscopy. The main advantage is that two-photon microscopy can produce fluorescence with wavelengths (far-red, infrared) that are less subjected to light scattering. This significantly enhances the capability of the microscope to penetrate deeper in dense tissue. Longer wavelengths are also less toxic for cells and moreover the probability of secondary photochemical reactions are significantly much lower than what observed in confocal microscopy.
References
[1] Hillman EMC. Optical brain imaging in vivo: techniques and applications from animal to man. Journal of biomedical optics. 2007;12(5):051402. doi:10.1117/1.2789693.
For more information :
https://en.wikipedia.org/wiki/Two-photon_absorption
https://www.microscopyu.com/techniques/multi-photon/multiphoton-microscopy
FAQ
Two-photon excitation microscopy is a specific imaging technique. It is based on the capability, under certain conditions, to excite one electron from its ground state by using two photons. The light used to create the excitation has a longer wavelength than the fluorescent light that is collected. This is different from single-photon methods, where the excitation light has more energy than the emitted light. The principle was originally predicted by M. Goeppert-Mayer in 1931 and later demonstrated in 1961\. Because longer wavelengths are used, the method is considered cell-friendly. It allows for the imaging of living specimens with few, if any, physiological alterations.
Most fluorescence microscopy relies on single-photon absorption. In this method, a fluorophore’s electron is excited by a single photon that has more energy than the light emitted during fluorescence. For instance, UV excitation is used to collect blue fluorescence. In two-photon microscopy, the electron is excited by two photons that have a longer wavelength than the resulting fluorescence emission. An electron is moved from the ground level by a first photon; it must then absorb a second photon while in its virtual state to reach the fully excited state. This is a second-order process, making it more difficult to obtain at low light intensities. Two-photon absorption only prevails over single-photon absorption when the photons are sufficiently dense.
The way this microscope illuminates a sample provides several benefits compared to confocal microscopy. The main advantage is the ability to produce fluorescence using longer wavelengths, such as far-red and infrared light. These wavelengths are less subject to light scattering. This property greatly enhances the microscope’s capability to penetrate deeper into dense tissue. Furthermore, longer wavelengths are less toxic to cells. The probability of secondary photochemical reactions, which cause photobleaching, is also much lower than what is observed in confocal microscopy. This makes the technique suitable for imaging living specimens with minimal physiological alteration.
The optical scheme of a two-photon microscope is similar to a standard laser scanner confocal microscope. Both technologies use a spotted light from a laser source and two galvanic mirrors to scan the sample. The main differences involve the illumination source and the lack of a sample pinhole. In confocal microscopy, a sample pinhole is required to filter fluorescence that originates away from the focal plane. This is not needed in two-photon microscopy. The physical principle dictates that fluorescence only occurs at the focal plane where photon density is high enough. This difference gives two-photon microscopy a better resolution in the z-axis. Additionally, confocal lasers use constant emission, while two-photon systems use pulsed lasers to concentrate photons in each pulse.