Introduction
Kidney Organoid and Microphysiological Kidney Chip Models, both have their own unique advantages and deficiencies. In the realm of drug research and discovery, they both have interesting applications.
In the study of the normal and sick human kidney, kidney organoids are considered significant instruments. Renal organoids have been effectively utilized to simulate glomerular and tubular disorders since the first reports of human pluripotent stem cell-derived human kidney organoids.
On the other hand, a kidney-on-a-chip, in synergy with Microphysiological System, can mimic the structural, mechanical, transport, absorptive, and physiological properties of the human kidney.
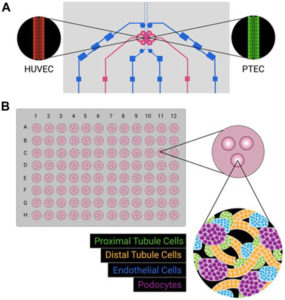
The review written below dives deep into the comparison of Kidney Organoid and Microphysiological Kidney Chip Models to Accelerate Drug Development and Reduce Animal Testing.
Abstract
The author states that,”Kidneys are critical for the elimination of many drugs and metabolites via the urine, filtering waste and maintaining proper fluid and electrolyte balance. Emerging technologies incorporating engineered three-dimensional (3D) in vitro cells culture models, such as organoids and microphysiological systems (MPS) culture platforms, have been developed to replicate nephron function, leading to enhanced efficacy, safety, and toxicity evaluation of new drugs and environmental exposures.
Organoids are tiny, self-organized three-dimensional tissue cultures derived from stem cells that can include dozens of cell types to replicate the complexity of an organ. In contrast, MPS are highly controlled fluidic culture systems consisting of isolated cell type(s) that can be used to deconvolute mechanisms and pathophysiology. Both systems, having their own unique benefits and disadvantages, have exciting applications in the field of kidney disease modeling and therapeutic discovery and toxicology.
In this review, we discuss current uses of both hPSC-derived organoids and MPS as pre-clinical models for studying kidney diseases and drug-induced nephrotoxicity. Examples such as the use of organoids to model autosomal dominant polycystic kidney disease, and the use of MPS to predict renal clearance and nephrotoxic concentrations of novel drugs are briefly discussed.
Taken together, these novel platforms allow investigators to elaborate critical scientific questions. While much work needs to be done, the utility of these 3D cell culture technologies has an optimistic outlook and the potential to accelerate drug development while reducing the use of animal testing.”
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
References
Chen WY, Evangelista EA, Yang J, Kelly EJ, Yeung CK. Kidney Organoid and Microphysiological Kidney Chip Models to Accelerate Drug Development and Reduce Animal Testing. Front Pharmacol. 2021 Jul 26;12:695920. DOI: 10.3389/fphar.2021.695920. PMID: 34381363; PMCID: PMC8350564.
FAQ
Two types of engineered 3D in vitro cell culture models have been developed. These are organoids and microphysiological systems, or MPS. Organoids are described as tiny, self-organised three-dimensional tissue cultures. They are derived from stem cells. These models can include dozens of cell types, which allows them to replicate the complexity of an organ. In contrast, MPS are defined as highly controlled fluidic culture systems. These systems consist of one or more isolated cell types. An MPS can be used to deconvolute, or separate, mechanisms and pathophysiology. Each system has its own distinct benefits and disadvantages. Both have applications in kidney disease modelling and therapeutic discovery.
Kidney organoids are regarded as significant instruments for studying the human kidney in both normal and diseased states. Human kidney organoids derived from human pluripotent stem cells have been effectively utilised. Their use includes simulating both glomerular and tubular disorders. As an example, organoids have been used to model autosomal dominant polycystic kidney disease. Organoids are defined as tiny, self-organised 3D tissue cultures that come from stem cells. They can contain dozens of cell types. This feature allows them to replicate the complexity of an organ. They have applications in the fields of kidney disease modelling, therapeutic discovery, and toxicology. These models help investigators to elaborate on critical scientific questions.
A kidney-on-a-chip model operates in synergy with a Microphysiological System, or MPS. This type of platform can mimic the structural and mechanical properties of the human kidney. Transport, absorptive, and other physiological properties can also be replicated. An MPS is a fluidic culture system that is highly controlled. It consists of one or more isolated cell types. These systems are often used to deconvolute mechanisms and pathophysiology. They are applied as pre-clinical models for studying drug-induced nephrotoxicity. For instance, MPS can be used to predict the renal clearance of new drugs. They are also applied to predict nephrotoxic concentrations of substances.
The kidneys are responsible for eliminating many drugs and metabolites from the body. They filter waste products and maintain the proper balance of fluid and electrolytes. Engineered three-dimensional in vitro cell culture models, such as organoids and MPS platforms, have been developed. These new models are intended to replicate nephron function. This replication is expected to lead to enhanced evaluation of new drugs. Efficacy, safety, and toxicity can be assessed. These platforms allow investigators to elaborate on scientific questions. The 3D cell culture technologies have an optimistic outlook. It is thought they have the potential to accelerate the drug development process. A further goal is to reduce the use of animal testing.