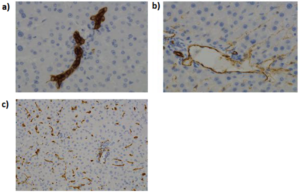
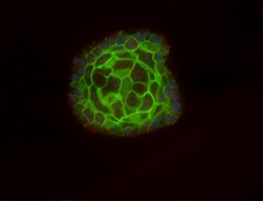
What is the organotypic culture ?
Organotypic culture is defined as the culture of an organ collected from an organism. It is one method allowing the culture of complex tissues or organs. It allows the preservation of the architecture of the cultured organ and most of its cellular interactions.
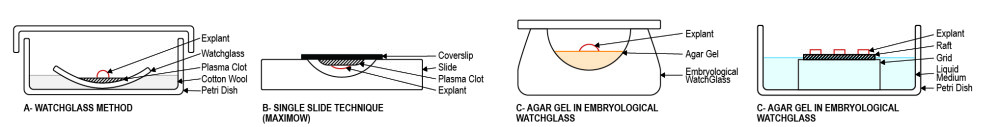
Several protocols have already been developed like agar methods, watch glass protocols, organ culture in media, and filter well inserts.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Historical background
While cell lines are one of the most often used experimental models by scientists, surprisingly, the culture of whole organs start a few decades before the first establishment of cell lines.
At the end of the 19th century, L. Loeb managed to cultivate the organs of rabbits. Quickly after, other authors report the culture of different organs collected from different organisms. In the meantime, the first establishment of cell lines has been done, slowing the development of organotypic culture. The HeLa cell line marked the beginning of the intensive utilization of such a system by the scientific community. For example, HeLa cells are now cited in more than 70000 articles and part of 11000 patents. MCF-7 cells (another classically used cell line) are cited in more than 23000 articles. It reflects the intensive use of cell lines in research.
Assets and drawbacks
The massive use of cell line by researchers comes from the need to have a robust, reproducible, cheap, and fast experimental model, that are key features of cell line cultures. Despite all of these advantages, some drawbacks appear over time. First, the genetic deviation/differences of cells from the same cell line between two samples lead to the loss of reproducibility and robustness while using cell lines as models. Second is the loss of certain cell functions because the culture conditions are driven by the growth and not by the expression of a tissue-like phenotype. An illustration of this is the metabolism in the liver and liver cell lines. This bias makes the extrapolation of cell lines data to human tricky or even unrealistic.
For a few years, the pressure of regulatory agencies to improve the risk assessment for humans has to lead to re-consider the use of organotypic cultures. Whereas they are more expensive, more complex, and time-consuming, they offer better extrapolation possibilities and are more relevant by taking into account the physiological environment. One of the best models is the culture of human organs, but the difficulties to handle such a system and to obtain such organs push researchers towards new systems offering both the advantages of cell cultures and organ culture.
From organotypic culture to 3D models
As mentioned above, there is now a need for developing new in vitro systems offering the advantages of both cell lines and organ culture. One of the solutions is the 3D cell cultures, eg. spheroids. The development of such a technique has to be based on the knowledge acquired with the two models. For more details on spheroïds, you can refer to this page.
References
Written by Pierre Gaudriault.
FAQ
Organotypic culture is a method defined by the cultivation of an organ that has been collected from an organism. It is a technique that permits the culture of intricate groups of cells or whole organs. A main characteristic of this method is the preservation of the original structure of the organ. Most of the cellular relationships within the organ are also maintained during the culture. Several different protocols have been created to perform this type of culture. Examples include methods using agar, watch glass protocols, and filter well inserts.
The culture of whole organs was reported at the end of the 19th century, before the first cell lines were established. The development of organotypic culture methods was slowed, however, once cell lines became available. The HeLa cell line, for instance, marked the start of intensive use of such systems by the scientific community. The widespread adoption of cell lines by researchers is related to the need for an experimental model with specific advantages. Scientists required a system that was dependable, consistent, inexpensive, and fast. These are all characteristics found in cell line cultures. This is reflected in the high number of articles and patents associated with lines like HeLa and MCF-7.
Despite the advantages of cell line models, certain drawbacks have appeared over time. One issue is the genetic deviation that can occur between two samples of the same cell line. This leads to a loss of consistency and dependability when these models are used. Another problem is the loss of certain cell functions. This happens because the culture conditions are designed to promote growth. They are not designed for the expression of a state that resembles a group of cells. An illustration of this is the difference in metabolism observed between a liver and a liver cell line. This distortion makes the application of cell line data to humans difficult or even unrealistic.
For a few years, pressure from regulatory agencies has led to a reconsideration of organotypic cultures. This is part of an effort to improve risk assessment for humans. While organotypic methods are more expensive, more intricate, and take more time, they offer better possibilities for applying data to humans. They are considered more applicable because they take the physiological environment into account. A need now exists for new systems that offer the advantages of both cell lines and organ culture. One of the solutions is the use of three-dimensional cell cultures, such as spheroids. The development of these newer techniques is based on the knowledge that was acquired from the two previous models.