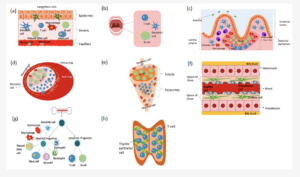
The interplay between adipose tissue and therapeutic efficacy is now recognised as a major determinant of clinical response [1].
Adipose tissue microenvironment constitutes an active metabolic and immunological niche that can modulate treatment outcomes.
As global obesity prevalence increases, elucidating how this specialised microenvironment governs tumour behaviour has become imperative [2]. Current evidence demonstrates that adipose-derived factors:
- reprogramme cancer-cell metabolism,
- reshape local and systemic immune responses, and
- condition therapeutic susceptibility and resistance profiles [3].
Consequently, a detailed characterisation of adipose–tumour crosstalk is expected to facilitate the development of refined, patient-specific therapeutic strategies [4].
The role of adipose tissue in cancer development
Adipose tissue is a multifunctional organ that integrates metabolic, endocrine, and immunological signalling pathways [1, 3].
Excess adiposity constitutes an established, dose-dependent risk factor for multiple malignancies [5].
Adipose-mediated tumour promotion is underpinned by several inter-related mechanisms:
- Pro-inflammatory secretome: sustained release of adipokines, cytokines, and growth factors that establish a tumour-permissive milieu [2, 3].
- Lipid provisioning: obesity-associated lipolysis supplies a continuous flux of fatty acids to rapidly proliferating cancer cells [6, 8].
- Local microenvironmental support: cooperative interactions among adipocytes, stromal fibroblasts, and infiltrating immune cells within the tumour microenvironment (TME) foster growth, invasion, and therapeutic resistance [4, 5].
Collectively, these systemic and tissue-level processes underscore the necessity of incorporating adipose biology into oncological risk models and intervention designs.
Learn more about our ready to use Adipose organoid plate.
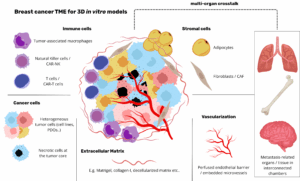
Understanding the microenvironment: key components
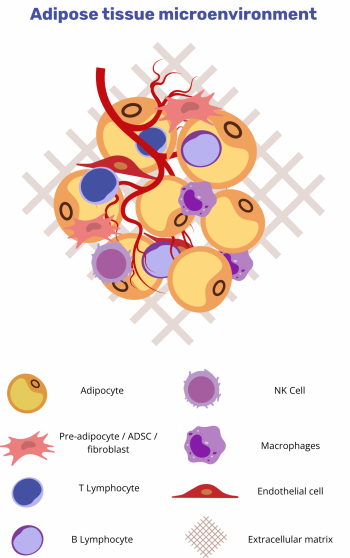
The adipose-tissue microenvironment encompasses distinct cell populations embedded within a dynamically remodelled extracellular matrix (ECM) [1].
Heterogeneity within this compartment dictates its tumour-modifying capacity.
Cellular constituents [3]
- Mature adipocytes / pre-adipocytes
- Fibroblasts and endothelial cells
- Immune subsets: macrophages, T lymphocytes, B lymphocytes, natural killer cells
Structural framework [4]
- Extracellular matrix: a protein-polysaccharide lattice regulating cell adhesion, migration, and growth-factor bioavailability; ECM composition and stiffness critically influence tumour progression and treatment response.
Bioactive mediators
- Adipokines: leptin (generally pro-tumorigenic) and adiponectin (often anti-
tumorigenic) [2, 5] - Cytokines: TNF-α, IL-6, IL-1β—central drivers of chronic inflammation [3]
- Growth factors: VEGF, IGF—facilitators of angiogenesis and proliferation [8]
The quantitative and qualitative balance of these mediators ultimately governs whether the microenvironment supports or constrains tumour growth.
Interaction between adipocytes and cancer cells
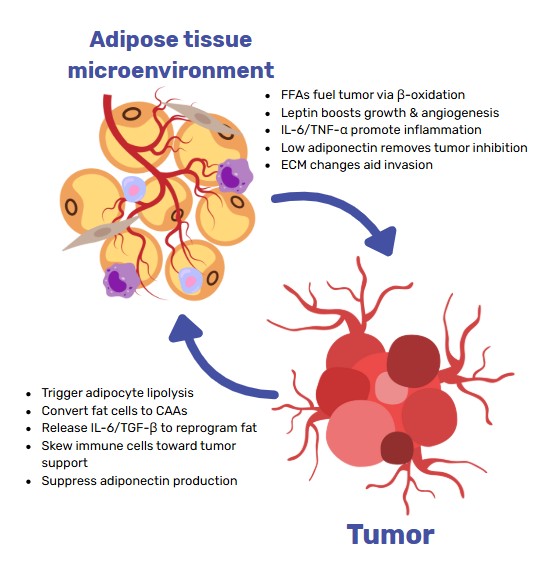
The interaction between adipocytes and cancer cells is a dynamic, reciprocal process mediated by intricate signalling networks [4]. Adipocytes influence tumour cells via secreted adipokines, cytokines and growth factors, as well as through direct cell–cell contact. Conversely, malignant cells reprogramme adipocyte metabolism and phenotype.
Adipocyte-derived cues potentiate malignant traits, while cancer cells induce a lipolytic, pro-inflammatory adipocyte phenotype.
Key mechanisms:
- Adipokine signalling. Leptin activates JAK/STAT, PI3K/AKT and MAPK pathways to promote proliferation, migration and angiogenesis, whereas
adiponectin exerts anti-tumour effects by constraining cell growth and inducing apoptosis [2, 5]. - Pro-inflammatory cytokine flux. TNF-α, IL-6 and IL-1β, produced by adipocytes and resident immune cells, trigger NF-κB and STAT3 activation, enhancing survival, invasion and metastatic potential [3, 4].
- Metabolic crosstalk. Cancer-induced lipolysis liberates free fatty acids that fuel β-oxidation and membrane biogenesis in tumour cells [6, 8].
- Phenotypic conversion. Tumour-secreted factors drive pre-adipocytes toward cancer-associated adipocytes (CAAs) with heightened lipolytic activity and cytokine output; novel in-vitro systems are now available to dissect these interactions [10].
The bidirectional exchange of signals and metabolites thus constitutes a critical driver of tumour progression and therapy resistance.
Adipose tissue and immune response in cancer
Adipose tissue harbours diverse immune subsets whose composition and functional state are profoundly altered in obesity [3, 5].
Obesity-associated immune remodelling skews the adipose microenvironment toward a pro-tumorigenic, immunosuppressive state.
Principal alterations
- Macrophage polarisation. Adipose-tissue macrophages adopt a pro-
inflammatory phenotype (TNF-α, IL-6, IL-1β) that supports tumour growth and dampens cytotoxic lymphocyte function [3, 5]. - T-cell repertoire shift. Increased Th1/Th17 frequencies and reduced
Treg/Th2 populations elevate IFN-γ and IL-17 levels, fostering chronic
inflammation and metastatic dissemination [3]. - Natural-killer-cell impairment. Adipose-derived leptin and free fatty acids diminish NK-cell cytotoxicity and cytokine production, weakening innate anti-tumour surveillance [3, 7].
Collectively, these changes not only promote oncogenesis but also attenuate the efficacy of immunotherapies that rely on robust effector responses.
Implications for cancer therapy: targeting the microenvironment
Traditional cytotoxic and radiation therapies chiefly address tumour cells; however, microenvironmental factors, including adipose tissue, critically influence therapeutic response and resistance patterns [1, 4].
Dual-target strategies that modulate both malignant cells and the adipose niche are emerging as rational interventions.
Therapeutic avenues
- Metabolic intervention. Inhibition of adipocyte lipolysis or cancer-cell fatty- acid oxidation restricts nutrient supply, curtailing tumour growth; engineered UCP1-upregulated adipocyte organoids exemplify this approach by out-competing tumours for glucose and lipids [6, 8, 11].
- Anti-inflammatory modulation. Pharmacological blockade of TNF-α, IL-6 or related cytokines, and re-polarisation of macrophages toward an anti-inflammatory phenotype, reduce pro-tumour signalling and may synergise with checkpoint inhibitors [3, 5].
- Endocrine targeting. Suppression of leptin signalling or elevation of adiponectin levels attenuates proliferative cues and can enhance immune competence, offering an adjunct to standard therapies [2, 5].
Integrating such microenvironment-focused strategies with conventional regimens holds promise for overcoming resistance and improving patient- specific outcomes.
Current research on adipose tissue and cancer treatment
A rapidly growing body of literature is delineating the molecular basis of adipose–tumour interactions and their therapeutic ramifications [1, 4].
1) Metabolic investigations
● Lipolysis blockade. Pharmacological or genetic inhibition of adipocyte lipolysis, as well as direct suppression of tumour fatty-acid uptake/β oxidation, reduces tumour burden and potentiates chemotherapeutic efficacy [6, 8].
● Pathway dissection. Isotope-tracing and multi-omics analyses are clarifying how specific lipid-handling enzymes (e.g., CPT1A, DGAT) govern proliferation and drug resistance. Inflammatory microenvironment studies
2) Inflammatory microenvironment studies
● Cytokine targeting. Neutralisation of TNF-α, IL-6 and related mediators dampens metastatic spread in pre-clinical models [3].
● Immune-cell reprogramming. Macrophage and T-cell plasticity within adipose tissue is being exploited to enhance checkpoint-inhibitor responsiveness [3, 5].
2) Endocrine-function research
● Adipokine signalling. Structure–function analyses of leptin and adiponectin receptors are identifying druggable nodes that modulate ERK, PI3K and AMPK pathways [2, 5].
Collectively, these studies are mapping a framework for microenvironment- informed therapeutic design.
Adipose tissue organoid and organ-on-chip models
Emerging bioengineered platforms like adipose tissue organoids and adipose tissue organ on chip systems are transforming how researchers study fat-tumor interactions, especially in the context of obesity-linked cancers. These advanced vitro models offer unprecedented insight into the adipose microenvironment and its role in cancer progression, drug resistance, and immune modulation.
Adipose Tissue Organoid — A 3D Model for Tumor–Fat Interaction
Adipose tissue organoids are 3D culture systems that replicate the structural and functional complexity of human fat tissue. These models preserve native lipid storage, adipokine signaling, and extracellular matrix composition, making them ideal for studying how adipose tissue influences cancer progression. When co-cultured with cancer cells, adipose tissue organoids reveal critical interactions such as fatty acid transfer, enhanced tumor invasiveness, and therapy resistance, especially in obese conditions. Compared to 2D cultures, organoids better mimic the tumor microenvironment, allowing high-throughput drug screening and mechanistic studies. Their human-relevant design and compatibility with patient-derived samples make adipose tissue organoids powerful tools for obesity-related cancer research.
Adipose Tissue Organ on Chip — Dynamic Modeling of the Tumor Microenvironment
The adipose tissue organ on chip integrates live fat tissue into a perfused microfluidic device, replicating blood flow, immune interactions, and tissue mechanics. These systems maintain mature adipocytes and recreate inflammatory and metabolic conditions seen in obesity. In cancer models, adipose tissue organ on chip platforms have shown how adipose-derived lipids fuel tumor growth and promote metastasis via hypoxia and vascular remodeling. Their tunable design allows precise control over oxygen, nutrients, and drug delivery, enabling real-time monitoring of tumor–fat–immune interactions. As scalable, human-relevant tools, adipose tissue organ on chip technologies are advancing cancer modeling and personalized drug testing. [11-16]
Future directions in cancer therapy and the adipose microenvironment
Integration of metabolic, inflammatory and endocrine insights is expected to drive the next generation of precision therapies [1, 5].
Strategic priorities
- Combination regimens. Concurrent targeting of tumour-intrinsic pathways and adipose-derived support systems; for example, pairing β oxidation inhibitors with anti-IL-6 antibodies, may achieve synergistic cytoreduction and delay resistance [8].
- Biomarker discovery. Circulating or imaging based signatures of adipokines, cytokines and adipose-tissue inflammation are under evaluation as predictors of treatment response and toxicity [5].
- Advanced diagnostics. Non-invasive modalities (e.g., PET tracers for adipose inflammation, hyperpolarised MRI for lipid flux) could enable real-time monitoring of microenvironmental dynamics and therapy adaptation [1].
Translational success will hinge on robust patient stratification and the rational pairing of microenvironmental targets with existing oncologic platforms.
Conclusion: the importance of considering adipose tissue in cancer therapy
The adipose-tissue microenvironment is now recognised as a critical determinant of oncogenesis, disease progression and therapeutic response [1, 4].
Failure to account for adipose-mediated metabolic, inflammatory and endocrine influences risks incomplete disease control [2, 3, 5].
Recent and ongoing investigations are elucidating actionable pathways (lipid metabolism, cytokine signaling, adipokine networks) that can be co-targeted alongside conventional modalities [6, 8]. Looking forward, microenvironment-tailored combination strategies, biomarker-guided patient selection and advanced imaging technologies promise to refine oncology practice and improve clinical outcomes [5, 8].
Integrating adipose biology into cancer-therapy paradigms therefore represents both a scientific imperative and an opportunity to deliver more effective, patient-specific care.
Resources
FAQ
Adipose tissue is now understood to be an active organ. It is not just for storage. It is involved in metabolic, endocrine, and immunological signaling. A clear link exists between excess body fat and a higher risk for many cancers. This connection is dose-dependent. The adipose microenvironment can change how well treatments work. This is because factors from adipose tissue can reprogramme how cancer cells get energy. These factors also reshape immune responses. Therapeutic resistance can be conditioned by these adipose-derived factors. As obesity prevalence increases, understanding this tissue’s role has become more necessary.
The adipose-tissue microenvironment is made of different cell populations. These populations are held within an extracellular matrix (ECM) that is dynamically remodelled. The specific composition of this compartment determines its effect on tumours. The cellular parts include mature adipocytes and pre-adipocytes. Fibroblasts and endothelial cells are also present. Several immune subsets, such as macrophages, T lymphocytes, and natural killer cells, are found there. The structural framework is the ECM. This is a lattice of protein and polysaccharide that regulates cell adhesion and migration. The ECM’s composition and stiffness are known to influence tumour progression and how treatments are received.
Bioactive mediators are present in the adipose microenvironment. These include adipokines, which are hormones from fat cells. Leptin is one such adipokine that is generally considered pro-tumorigenic. Adiponectin is another, and it is often found to be anti-tumorigenic. Cytokines are also present. These are central drivers of chronic inflammation. Specific examples include TNF-α, IL-6, and IL-1β. A third category is growth factors, such as VEGF and IGF. These substances are facilitators of new blood vessel formation (angiogenesis) and cell proliferation. The quantitative and qualitative balance of these different mediators is what governs if the microenvironment will support or constrain the growth of a tumour.
The interaction between adipocytes and cancer cells is a reciprocal process. Complex signalling networks are used for this communication. Adipocytes are known to influence tumour cells. This is done via secreted adipokines, cytokines, and growth factors. In the other direction, malignant cells reprogramme the metabolism and phenotype of adipocytes. A lipolytic and pro-inflammatory phenotype is induced in the fat cells by the cancer cells. This bidirectional exchange of signals and metabolites is a driver of tumour progression. It also contributes to therapy resistance. New in-vitro systems are now available. These systems are used to dissect these specific interactions.
Metabolic crosstalk is one of the main mechanisms of interaction. Cancer cells are able to induce lipolysis in nearby fat cells. This process liberates free fatty acids. These fatty acids are then used by the tumour cells. They fuel β-oxidation and are used for membrane biogenesis. This provides a continuous supply of fatty acids to the rapidly proliferating cancer cells. In addition to this energy supply, tumour-secreted factors can also cause a phenotypic conversion. Pre-adipocytes are driven to become cancer-associated adipocytes, or CAAs. These CAAs are cells with heightened lipolytic activity. They also have a higher cytokine output. This change helps to sustain the metabolic provisioning for the tumour.
Adipose tissue contains diverse immune subsets. The composition and functional state of these cells are profoundly altered in obesity. This immune remodelling associated with obesity skews the adipose microenvironment. It is changed toward a pro-tumorigenic and immunosuppressive state. Adipose-tissue macrophages are one example. They adopt a pro-inflammatory phenotype. This state supports tumour growth. It also dampens the function of cytotoxic lymphocytes. The T-cell repertoire is also shifted. An increase in Th1/Th17 frequencies and a reduction in Treg/Th2 populations is seen. This shift fosters chronic inflammation. These changes can weaken the efficacy of immunotherapies that require robust effector responses.
Natural killer (NK) cell function can be impaired by the adipose microenvironment. Adipose-derived substances, such as leptin and free fatty acids, are responsible for this effect. The cytotoxicity of the NK-cells is diminished. Cytokine production by the NK-cells is also lessened. This process weakens the innate anti-tumour surveillance that NK-cells normally provide. This is part of a larger shift in the immune landscape. In obesity, the adipose microenvironment becomes pro-tumorigenic and immunosuppressive. This alteration not only promotes oncogenesis. It also attenuates the effectiveness of immunotherapies. These therapies depend on robust effector responses from cells like NK-cells.
Dual-target strategies are being investigated as rational interventions. These strategies modulate both the malignant cells and the adipose niche. One therapeutic avenue is metabolic intervention. This involves the inhibition of adipocyte lipolysis. Cancer-cell fatty-acid oxidation can also be restricted. This curtails the nutrient supply to the tumour. Engineered adipocyte organoids with upregulated UCP1 are one example of this approach. Another avenue is anti-inflammatory modulation. This can be done by pharmacological blockade of cytokines like TNF-α or IL-6. Macrophages can also be re-polarised toward an anti-inflammatory state. Endocrine targeting, such as suppressing leptin signalling, is also being studied.
Adipose tissue organoids are three-dimensional culture systems. They are used to replicate the structural and functional complexity of human fat tissue. Native lipid storage, adipokine signaling, and the composition of the extracellular matrix are preserved in these models. This makes them well-suited for studying how adipose tissue influences cancer progression. When they are co-cultured with cancer cells, specific interactions are revealed. These include fatty acid transfer from fat to tumour. Enhanced tumour invasiveness and therapy resistance are also seen, especially in conditions meant to mimic obesity. These 3D models are considered more representative of the tumour microenvironment than 2D cultures.
The adipose tissue organ-on-chip integrates live fat tissue into a perfused microfluidic device. This setup is used to replicate blood flow. Immune interactions and tissue mechanics are also replicated. These systems are able to maintain mature adipocytes. They can also recreate the inflammatory and metabolic conditions that are seen in obesity. In cancer models, these platforms have been used to show how adipose-derived lipids fuel tumour growth. Metastasis promotion via hypoxia and vascular remodeling has also been shown. The design is tunable. Precise control over oxygen, nutrients, and drug delivery is allowed. Real-time monitoring of interactions is also enabled.