Triple-negative breast cancer (TNBC) is a distinct pathological subtype defined by the absence of estrogen receptor (ER), progesterone receptor (PR), and HER2 amplification . This immunohistochemical profile excludes endocrine and HER2-directed therapies, leaving cytotoxic chemotherapy as the principal systemic option. TNBC accounts for approximately 15–25% of all breast cancers and is characterized by early recurrence, visceral dissemination, and a shorter overall survival relative to other breast cancer subtypes(1). Molecular analyses have revealed considerable diversity within TNBC. Transcriptomic studies divide it into multiple clusters including basal-like 1 and 2, mesenchymal, immunomodulatory, and luminal androgen receptor (LAR) groups, each displaying distinct biological features and therapeutic sensitivities. Basal-like TNBC often shows transient responsiveness to chemotherapy followed by relapse, whereas mesenchymal variants display intrinsic resistance and enhanced invasiveness. Despite extensive profiling, no unifying target has emerged, leaving most TNBCs dependent on broad cytotoxic regimens. Clinical outcomes remain unsatisfactory, with roughly 40 % of patients experiencing relapse within three years of diagnosis and limited options at recurrence. Recent therapeutic advances such as PARP inhibition in BRCA1/2-mutated tumors and immune checkpoint blockade in PD-L1–positive disease benefit only a subset of patients. These limitations highlight the urgent need for improved experimental models that can accurately reproduce TNBC biology and predict drug efficacy. In this context, TNBC organoids have emerged as promising preclinical tools, bridging the gap between conventional two-dimensional cultures and complex in vivo systems. By replicating tumor heterogeneity, architecture, and microenvironmental factors, breast cancer organoids and particularly TNBC organoids offer a powerful platform to study tumor progression, drug response, and resistance mechanisms with higher translational fidelity (2).
Learn more about our ready to use breast cancer organoid models.
Barriers to treatment and limitations of conventional experimental systems
The management of TNBC is complicated by its aggressive phenotype, rapid metastatic progression, and extensive intratumoral variation . Resistance to chemotherapy remains a defining challenge, emerging through clonal selection, epigenetic adaptation, and metabolic reprogramming. Identifying treatments that suppress both primary tumor growth and metastatic seeding requires models that faithfully represent the three-dimensional architecture and cellular heterogeneity of the disease.
Conventional two-dimensional (2D) cultures fall short of this requirement. TNBC cell lines maintained on flat plastic surfaces lose polarity and microenvironmental context. They exhibit altered gene expression, uniform nutrient exposure, and reduced extracellular matrix signaling, conditions that diverge markedly from in vivo tumors. Drug responses in 2D systems often overestimate efficacy because diffusion barriers, stromal interactions, and hypoxic regions are absent.
Animal models provide greater structural complexity but introduce interspecies differences in immune function, metabolism, and stroma composition that obscure interpretation. Patient-derived xenografts (PDXs) retain human tumor architecture but require immunodeficient hosts and long engraftment periods, limiting scalability. These constraints hinder systematic drug screening and delay translational progress .
The need for reliable and physiologically relevant preclinical models has led to the adoption of three-dimensional (3D) in vitro cultures and particularly breast cancer organoids and TNBC organoids which reproduce essential histological and molecular properties of the disease. These include cell line–derived organoids, patient-derived organoids (PDOs), and microfluidic tumor-on-chip systems, each representing distinct levels of biological complexity and experimental precision.
3D breast cancer organoids as tools to investigate TNBC
Three-dimensional organoid systems offer an improved representation of tumor physiology. They recreate the architecture, cell–matrix communication, and biochemical gradients of solid tumors more effectively than monolayers. In the context of TNBC, organoid cultures allow detailed examination of tumor growth patterns, drug responses, and cell-cell interactions in a format compatible with quantitative imaging and high-content screening(3).
Cell line–derived breast cancer organoids in triple-negative breast cancer
Cell line–derived breast cancer organoids have become standard in the study of triple-negative breast cancer (TNBC). These cultures originate from established TNBC cell lines such as MDA-MB-231 and BT-20, grown under non-adherent or matrix-supported conditions. They reproduce the dense cellular organization and nutrient gradients typical of solid tumors, permitting the study of growth, invasion, and therapy resistance under more relevant spatial and biochemical conditions than monolayer culture.
Huang et al. (2020) developed MDA-MB-231 TNBC organoids in a suspension-based format(4). The model formed compact aggregates with distinct polarity and oxygen-deprived cores. Expression of vimentin and N-cadherin increased, consistent with an epithelial–mesenchymal transition profile. Drug testing indicated that cytotoxic activity of common agents was reduced in 3D compared to 2D, confirming that spatial organization alters treatment response. Ncube et al. (2023) obtained comparable findings using BT-20 cell line organoids prepared with a liquid-overlay method(5). The organoids developed dense architecture, visible hypoxia, and reinforced adherens junctions. Doxorubicin sensitivity dropped sharply, with the half-maximal inhibitory concentration rising from around 0.3 µM in monolayer to above 6 µM in 3D, showing how structure influences diffusion and cell survival.
Studies by Casali et al. (2022, 2024) extended the use of TNBC organoids to microenvironmental and co-culture models. Under reduced oxygen, MDA-MB-231 organoids exhibited increased invasiveness through αvβ3-integrin signaling. Blocking this pathway curtailed migration and diminished expression of mesenchymal markers. When endothelial cells were introduced into the system, forming mixed tumor–endothelium organoids, inhibition of αvβ3 disrupted endothelial plasticity and restrained TNBC infiltration into the matrix. These experiments established that cell line–derived breast cancer organoids can reproduce tumor–vascular interactions and environmental stresses relevant to metastatic progression(6,7).
Methodological advances have improved reproducibility and analytical power. A 2024 study in Tissue Engineering Part C (SAGE) used a hanging-drop technique combined with Matrigel to generate uniform BT-20 and MDA-MB-231 organoids suitable for quantitative imaging and transcriptomics. The transcriptome of cells in 3D differed markedly from 2D cultures, with enrichment in extracellular matrix remodeling, hypoxia adaptation, and apoptosis-related pathways. The protocol yielded stable organoid morphology across replicates, addressing variability often encountered in suspension cultures and allowing consistent comparison between compounds(8).
Across these reports, chemotherapeutic agents such as epirubicin, cisplatin, and docetaxel show lower efficacy in breast cancer organoids than in 2D models. Drug gradients, altered metabolism, and stress adaptation within organoids correspond to features seen in clinical TNBC. This makes such models suitable for ranking compounds, optimizing dose ranges, and identifying therapies capable of overcoming structural resistance.
Beyond pharmacological evaluation, cell line–derived organoids permit exploration of cell–matrix signaling and adaptation to environmental conditions. The controlled genetic background of cell lines facilitates mechanistic studies where a single pathway can be perturbed and monitored without the variability inherent to patient samples. This reproducibility explains their integration into experimental pipelines preceding patient-derived organoids or animal models.
Patient-derived breast cancer organoids (PDOs)
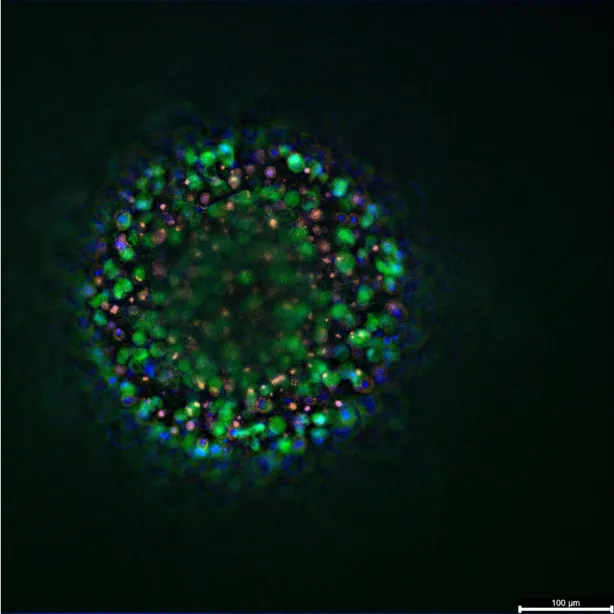
PDOs originate from surgical specimens or biopsies and preserve the histological and genetic diversity of the original tumor . When TNBC samples are dissociated and embedded in extracellular matrix gels, they self-organize into multicellular structures maintaining epithelial polarity, desmoplastic stroma remnants, and authentic mutational profiles. TNBC organoids generated through this method retain the phenotypic markers of their parental tumors, including cytokeratin 5/6, vimentin, and EGFR expression .
A critical feature of PDOs is their predictive capacity for therapy response. Ryu et al. (2025) for instance compared the ex vivo sensitivity of TNBC PDOs to clinical outcomes and found strong concordance between organoid drug responses and patient treatment results(9). In multiple independent studies, organoids that were resistant to taxanes or anthracyclines in vitro corresponded to non-responding tumors in patients, confirming the translational relevance of the model.
Beyond reproducing sensitivity patterns, PDOs help elucidate mechanisms of resistance. Sequential PDOs established from the same patient before and after chemotherapy reveal gene expression alterations associated with acquired tolerance. Comparative RNA sequencing identified enrichment of epithelial–mesenchymal transition signatures and activation of DNA repair pathways in resistant TNBC organoids .
PDOs also allow stratification of TNBC subtypes according to genomic and transcriptomic features correlated with treatment outcome. For example, organoids harboring FGFR3 mutations display selective sensitivity to FGFR inhibitors, while those with TP53 loss exhibit altered DNA-damage responses(10). Establishing large TNBC organoid biobanks enables comparative testing across dozens of patient backgrounds, providing a reference framework for precision oncology.
Although PDOs reproduce epithelial heterogeneity, they initially lack immune and stromal compartments. Co-culture approaches now incorporate autologous peripheral blood mononuclear cells or fibroblasts to rebuild aspects of the tumor microenvironment. Immune-competent TNBC organoid systems permit evaluation of checkpoint inhibitors and cytokine-modulating drugs under controlled conditions. These integrated cultures have demonstrated interactions between tumor and immune cells that influence sensitivity to PD-1 blockade, reflecting the immunogenic heterogeneity observed clinically.
Compared with animal models, PDOs are faster to establish, more scalable, and retain direct patient relevance. Drug evaluation using breast cancer organoids can be completed within weeks, allowing near-real-time therapeutic assessment. Their high fidelity and clinical correlation position PDOs as indispensable tools in both translational research and individualized therapy selection for TNBC.
Tumor-on-chip systems
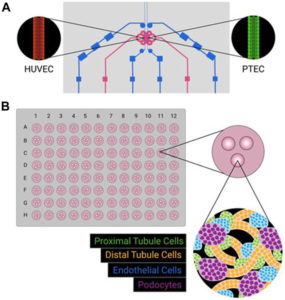
Microfluidic tumor-on-chip models extend organoid technology by recreating tissue interfaces and continuous fluid perfusion. These devices integrate tumor, stromal, and vascular components within a transparent polymer scaffold containing microchannels through which culture medium flows. The setup allows precise control of nutrient gradients, oxygenation, and shear stress, mimicking physiological conditions more closely than static cultures.
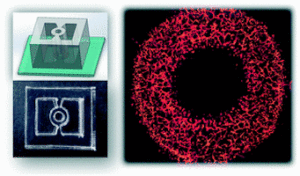
In TNBC research, tumor-on-chip devices have been used to investigate invasion, vascular permeability, and drug transport. Sigdel et al. (2023) developed a microfluidic chip containing a central 3D TNBC tumor flanked by endothelial channels lined with human umbilical vein endothelial cells (HUVECs) . When paclitaxel was delivered through these vascular channels, tumor reduction occurred alongside extensive endothelial damage and barrier leakage(11). In contrast, treatment with the experimental thienopyrimidine compound TPH104c suppressed TNBC growth without compromising endothelial integrity and prevented tumor cell intravasation . This dual assessment of efficacy and vascular toxicity highlights the advantage of dynamic perfused models.
Tumor-on-chip systems also permit visualization of metastatic behavior. TNBC cells can be tracked as they invade through extracellular matrix, penetrate endothelial barriers, and migrate into perfused channels, recapitulating early metastasis steps. Connecting breast tumor chambers to downstream “organ” compartments such as bone or lung microenvironments allows study of organotropic colonization, a hallmark of TNBC dissemination .
Beyond metastasis research, these platforms are used to assess anti-angiogenic and immunomodulatory therapies. Integration of immune cells into the microfluidic environment enables analysis of cytokine gradients and immune infiltration under flow conditions. Drug candidates can be tested not only for direct cytotoxicity but also for their influence on vascular stability and immune cell recruitment.
Microfluidic TNBC models are highly quantitative. Parameters such as flow rate, permeability, and mechanical stiffness can be adjusted precisely. Imaging within the device allows real-time tracking of tumor growth, apoptosis, and invasion at single-cell resolution. These attributes make tumor-on-chip systems valuable for dissecting mechanisms underlying metastatic potential and for screening compounds targeting tumor–stroma interactions.
Conclusion
Triple-negative breast cancer remains one of the most aggressive forms of breast malignancy, characterized by high heterogeneity and limited therapeutic options. Progress in understanding and treating this disease depends on experimental systems capable of replicating its complex biological context.
Three-dimensional organoid models have become indispensable in this effort. Cell line–derived breast cancer organoids provide standardized, reproducible systems for mechanistic studies and compound screening. Patient-derived breast cancer organoids (PDOs) retain the genetic and phenotypic heterogeneity of TNBC, allowing individualized drug testing and discovery of resistance pathways. Tumor-on-chip devices introduce dynamic physiological conditions and multicellular organization, enabling direct observation of metastasis and drug transport.
Collectively, these models form a continuum of experimental complexity that bridges conventional in vitro assays and patient studies. Expanding TNBC organoid biobanks, refining co-culture protocols, and integrating microfluidic technology are expected to improve translational precision. Through these approaches, breast cancer organoids and TNBC organoids are reshaping preclinical research and guiding the search for more effective therapies against this challenging disease.
References
- Xiong X, Zheng LW, Ding Y, Chen YF, Cai YW, Wang LP, et al. Breast cancer: pathogenesis and treatments. Signal Transduct Target Ther. 19 févr 2025;10(1):49.
- Chen Z, Liu Y, Lyu M, Chan CH, Sun M, Yang X, et al. Classifications of triple-negative breast cancer: insights and current therapeutic approaches. Cell Biosci. 1 févr 2025;15(1):13.
- Bittman-Soto XS, Thomas ES, Ganshert ME, Mendez-Santacruz LL, Harrell JC. The Transformative Role of 3D Culture Models in Triple-Negative Breast Cancer Research. Cancers. 13 mai 2024;16(10):1859.
- Huang Z, Yu P, Tang J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. juin 2020;Volume 13:5395‑405.
- Ncube KN, Jurgens T, Steenkamp V, Cromarty AD, Van Den Bout I, Cordier W. Comparative Evaluation of the Cytotoxicity of Doxorubicin in BT-20 Triple-Negative Breast Carcinoma Monolayer and Spheroid Cultures. Biomedicines. 19 mai 2023;11(5):1484.
- Casali BC, Baptista MP, Pachane BC, Cortez AA, Altei WF, Selistre-de-Araújo HS. Blockage of αvβ3 integrin in 3D culture of triple-negative breast cancer and endothelial cells inhibits migration and discourages endothelial-to-mesenchymal plasticity. Biochem Biophys Rep. juill 2024;38:101686.
- Casali BC, Gozzer LT, Baptista MP, Altei WF, Selistre-de-Araújo HS. The Effects of αvβ3 Integrin Blockage in Breast Tumor and Endothelial Cells under Hypoxia In Vitro. Int J Mol Sci. 3 févr 2022;23(3):1745.
- Olvera-Valencia M, Garcia-Castillo V, Ramos-Payan R, Aguilar-Medina M, Trujano-Camacho S, López-Saavedra A, et al. Development of a reliable method for human triple-negative breast cancer organotypic culture: Improving imaging and genomic studies in 3D cultures.
- Ryu S, Kim HS, Lee S, Yoon SH, Baek M, Park AY, et al. Anti-cancer drug sensitivity testing and preclinical evaluation of the anti-cancer potential of WEE1 inhibitor in triple-negative breast cancer patient-derived organoids and xenograft models. Breast Cancer Res. 23 juin 2025;27(1):113.
- Sheikh M, Bagga H, Bhojwani Y, Telrandhe U. The role of 3D culture models and advanced chromatography in exosome research for triple-negative breast cancer. J Egypt Natl Cancer Inst. 27 sept 2025;37(1):67.
- Sigdel I, Ofori-Kwafo A, Heizelman RJ, Nestor-Kalinoski A, Prabhakarpandian B, Tiwari AK, et al. Biomimetic on-chip assay reveals the anti-metastatic potential of a novel thienopyrimidine compound in triple-negative breast cancer cell lines. Front Bioeng Biotechnol. 28 sept 2023;11:1227119.
FAQ
Triple-negative breast cancer (TNBC) is a specific pathological type. It is identified by the lack of three receptors: estrogen receptor (ER), progesterone receptor (PR), and HER2 amplification. This profile means that endocrine and HER2-targeted treatments are not effective. Cytotoxic chemotherapy is left as the main systemic treatment. This cancer type represents about 15–25% of breast cancers. It is known for early relapse, spreading to internal organs, and shorter survival compared to other types. Molecular studies show it is very diverse, with groups like basal-like, mesenchymal, and luminal androgen receptor (LAR). These groups have different biological characteristics and responses to therapy. This variation makes finding a single treatment target difficult.
Conventional two-dimensional (2D) cultures do not meet the requirements for studying TNBC effectively. When TNBC cell lines are grown on flat plastic, they lose their polarity and microenvironmental context. This leads to altered gene expression. The cells also experience uniform nutrient exposure and less extracellular matrix signalling. These conditions are very different from how tumours grow inside the body. Drug testing in 2D systems often overestimates how well a treatment works. This is because important factors like diffusion barriers, interactions with stromal cells, and low-oxygen regions are missing. These shortcomings mean 2D models are not reliable for predicting drug efficacy in patients.
Animal models present more structural complexity than 2D cultures. Yet, they also introduce challenges that can make results difficult to interpret. Interspecies differences in immune function, metabolism, and the composition of stromal tissue exist. These differences can obscure the true biological processes of human TNBC. Patient-derived xenografts (PDXs) are one type of animal model that keeps the human tumour structure. However, PDXs must be grown in immunodeficient hosts. They also require long periods for engraftment, which limits their scalability. These constraints make systematic drug screening difficult and can delay the progress of translational research.
Cell line-derived breast cancer organoids are frequently used in TNBC research. These cultures start from established TNBC cell lines, like MDA-MB-231 or BT-20. They are grown in non-adherent conditions or supported by a matrix. The organoids reproduce the dense cell organisation and nutrient gradients found in solid tumours. This allows the study of growth, invasion, and therapy resistance under more realistic spatial conditions than flat cultures. For example, studies show that cytotoxic drug activity is often reduced in these 3D models compared to 2D cultures. This finding confirms that spatial organisation changes treatment response. These models are also used for co-culture experiments, such as introducing endothelial cells to study tumour–vascular interactions.
Patient-derived organoids, or PDOs, originate directly from surgical specimens or biopsies taken from a patient. They are valued because they preserve the histological and genetic diversity of the original tumour. When TNBC samples are processed and placed in extracellular matrix gels, they self-organise into multicellular structures. These structures maintain epithelial polarity and remnants of the desmoplastic stroma. They also keep the authentic mutational profiles of the tumour. TNBC organoids created this way retain the specific phenotypic markers of their parent tumours. These markers can include cytokeratin 5/6, vimentin, and EGFR expression. This high fidelity to the original patient material makes PDOs very useful for translational research.
A key capability of PDOs is their predictive capacity for therapy response. Research has been conducted comparing the ex vivo sensitivity of TNBC PDOs to actual clinical outcomes. These comparisons found a strong agreement between how the organoids responded to drugs and the results seen in patients. In several separate studies, organoids that were resistant to taxanes or anthracyclines in the laboratory corresponded to tumours that did not respond in patients. This confirms the translational relevance of the model. Drug evaluation using these organoids can be finished within weeks. This speed allows for therapeutic assessment that is almost in real-time, positioning PDOs as tools for individualized therapy selection.
Patient-derived organoids initially reproduce epithelial heterogeneity but often lack the immune and stromal compartments of a tumour. To address this, co-culture approaches are now used. These methods incorporate autologous (originating from the same patient) peripheral blood mononuclear cells or fibroblasts. This process helps to rebuild parts of the tumour microenvironment. Immune-competent TNBC organoid systems created in this way permit the evaluation of checkpoint inhibitors and cytokine-modulating drugs. These evaluations can be done under controlled conditions. Such integrated cultures have shown interactions between tumour and immune cells that affect sensitivity to PD-1 blockade. This reflects the immunogenic variation seen in clinical situations.
Microfluidic tumour-on-chip models are an extension of organoid technology. They recreate tissue interfaces and continuous fluid perfusion. These devices are made of a transparent polymer scaffold with microchannels. Tumour, stromal, and vascular components are integrated within this scaffold, and culture medium flows through the channels. This design allows exact control of nutrient gradients, oxygenation, and shear stress. In TNBC research, these devices are used to investigate invasion, vascular permeability, and drug transport. For example, drugs can be delivered through integrated vascular channels to assess both tumour reduction and any damage to the endothelium. These systems also permit visualization of metastatic behaviours, such as cells invading endothelial barriers.
The different three-dimensional models form a continuum of experimental complexity. Each model serves a different purpose in TNBC research. Cell line–derived breast cancer organoids provide standardized, reproducible systems. These are well-suited for mechanistic studies and for initial compound screening. Patient-derived breast cancer organoids (PDOs) retain the genetic and phenotypic variation of the patient’s TNBC. This allows for individualized drug testing and the discovery of resistance pathways. Tumour-on-chip devices add dynamic physiological conditions and multicellular organisation. These are used for direct observation of metastasis and drug transport. Collectively, these models connect simple in vitro assays with studies involving patients. Expanding TNBC organoid biobanks and refining these technologies are expected to improve translational precision.