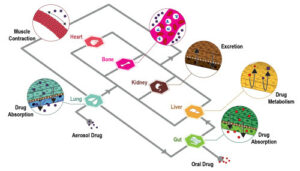
Context
Solid tumors are characterized by areas of hypoxic tissue heterogeneously distributed inside the tumor mass. Hypoxia triggers tumor aggressivity, as it tends to select for increased mutation rates, angiogenesis, and invasion capacity and metastasis. Additionally, hypoxia generates drug resistance and correlates with bad prognosis [1].
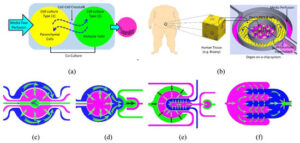
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Hypoxia, vascularization and metastasis
Vascularization and hypoxia
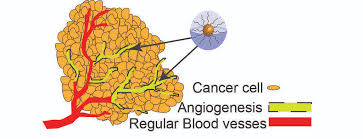
Hypoxic growth is a hallmark of solid tumors. Paradoxically, hypoxia occurs in the context of a highly vascularized environment which enables tumor growth by providing nutrients and signaling factors secreted in the tumor microenvironment (read more: Tumor Microenvironment). Nevertheless, this vascularization is abnormal with blind ends and occlusions, and vessels cannot properly oxygenate the neighboring cells. As a consequence, there is an imbalance between oxygen consumption and supply [1] [2] and tumors remain hypoxic despite angiogenesis [4].
Hypoxia contributes to tumor aggressiveness through several mechanisms. First, by inducing changes in gene expression, mainly through the HIF family of transcription factors (hypoxia-induced Factor) which promote the expression of angiogenic factors, growth factors and glycolytic enzymes which will remodel the extra cellular matrix (ECM) [3][4]. These changes in gene expression lead to hypoxic adaptation, tumor growth and drug resistance. More details about HIF-1 role in hypoxia can be seen in the following video:
Second, hypoxia results in the local recruitment of endothelial progenitor cells and mesenchymal stem cells (MSC) (see Link to TME Scientific Note), which will further contribute to hypoxia through the secretion of VEGF [5]. Additionally, hypoxia has been linked to DNA damage. Cycles of hypoxia and reoxygenation can lead to ROS (reactive oxygen species) production, oxidative stress and DNA damage. At the same time, there is a hypoxia -induced downregulation of DNA repair pathways. All these factors contribute to the genomic instability [6].
Cancer cell adaptation to hypoxia through transcriptomic and genetic changes induces selection of more malignant clones resistant to hypoxia and with more invasive phenotype.
Hypoxia and metastasis
Hypoxia can allow tumor cells to escape from the hypoxic environment, through neovascularization and metastasis [4]. Metabolism in the inner parts of solid tumor will be affected by the hypoxic conditions: glycolytic pathways will be activated in a metabolic change known as the cancer metabolic shift. Acidic pH is generated in the extracellular environment following this metabolic shift, while pH inside tumor cells remains neutral. Acidity has been shown to promote cell migration through extra cellular matrix (ECM) degradation. H+ diffuses from the TME to adjacent tissues, contributing the ECM degradation and facilitating invasion to these adjacent tissues, while invasion doesn’t occur in areas with normal pH [3],[7].
Examples of HIF factors (hypoxia induced factors) involved in metastasis are [4]:
- LOX (Lysyl oxidase). It is induced by hypoxia and induces modifications in the extracellular matrix (ECM). LOX cross-links collagen and elastin at the ECM, and in healthy tissue it has a normal function in development and tissue remodeling. In tumor cells LOX acts as a pro-metastatic factor when secreted by tumor cells, facilitating ECM remodeling and cancer cell invasion. LOX expression is correlated with disease progression and metastasis.
- CTGF (Connective tissue growth factor). It is and extracellular matrix protein induced by hypoxia. Expression levels are linked to tumor growth and metastasis.
Hypoxic growth and drug sensitivity
Hypoxia can have a critical impact in the efficacity of anticancer therapies. Hypoxic regions are linked to necrosis and quiescence, and drugs targeting rapidly growing cells will fail to target quiescent cancer cells. This inner quiescent part of tumors is resistant to radiotherapy and chemotherapy targeting proliferating cells [2]. For instance, hypoxic zones are insensitive to drugs targeting the p53-mediated apoptosis. Drugs which target cancer cells by generating DNA damage through contact with oxygen (free radicals) will fail to target hypoxic tumor cells too. Drug delivery to hypoxic parts of the tumor is also impaired through abnormal blood vessels in the tumor vascularized zone. Finally, hypoxia can induce transcriptional upregulation of drug resistance genes. All these examples show how the hypoxic tumor microenvironment can have a role in drug sensitivity [2],[8].
On the other hand, hypoxia has been used to favor anticancer therapies, through different strategies like prodrugs activated upon hypoxia. Also, it has been used as a drug target itself, with drugs targeting the HIF 1 transcription factor [2].
Mimicking real oxygen exposure of cancer cells in in vitro assays is critical to asses drug efficacity and tumor growth. Experimental methods allowing for dynamic oxygen control are needed, together with cell culture methods recapitulating the complex tumor environment (see Scientific Note 3D cell culture and anticancer drugs).
References
[1] Vaupel P. and Mayer A. Hypoxia in cancer: significance and impact on clinical outcome.Cancer Metastasis Rev (2007) https://www.ncbi.nlm.nih.gov/pubmed/27526128
[2] Brown JM, William WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer (2004) https://www.ncbi.nlm.nih.gov/pubmed/15170446.
[3] Castells M. et al. Implication of Tumor Microenvironment in Chemoresistance: Tumor-Associated Stromal Cells Protect Tumor Cells from Cell Death. Int. J. Mol. Sci. 2012 https://www.ncbi.nlm.nih.gov/pubmed/22949815
[4] Finger EC. and Giaccia AJ. Cancer Metastasis Rev. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease (2010) https://www.ncbi.nlm.nih.gov/pubmed/20393783
[5] Li HC et al. Tumor microenvironment: The role of the tumor
stroma in cancer. J Cell Biochem. 2007 https://www.ncbi.nlm.nih.gov/pubmed/17226777
[6] Bindra RS. and Glazer PM. Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res (2015) https://www.ncbi.nlm.nih.gov/pubmed/15603753
[7] Estrella V. et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Research (2013) https://www.ncbi.nlm.nih.gov/pubmed/23288510
[8] Jo Y. et al. Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-mimicking In Vitro Models in Anti-Cancer Drug Development. Theranostics. 2018. https://www.ncbi.nlm.nih.gov/pubmed/30555545