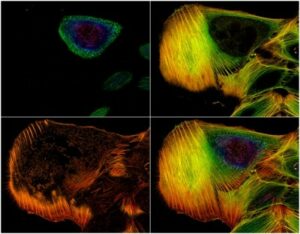
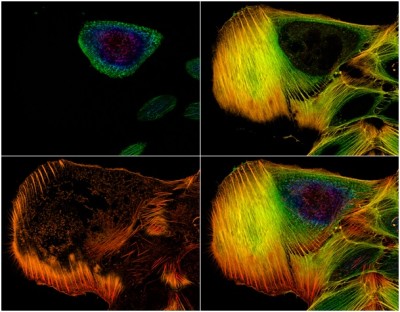
Introduction to fluorescence microscopy
Fluorescence is a natural phenomenon in which following the absorption of light by a molecule (fluorophore), the molecule almost immediately emits a light (with a longer wavelength). The phenomenon has been deeply exploited in many scientific fields, here comprised microscopy. Fluorescence microscopy relies on the capability to excite and selectively collect the emission of samples previously labeled with specific fluorophores.
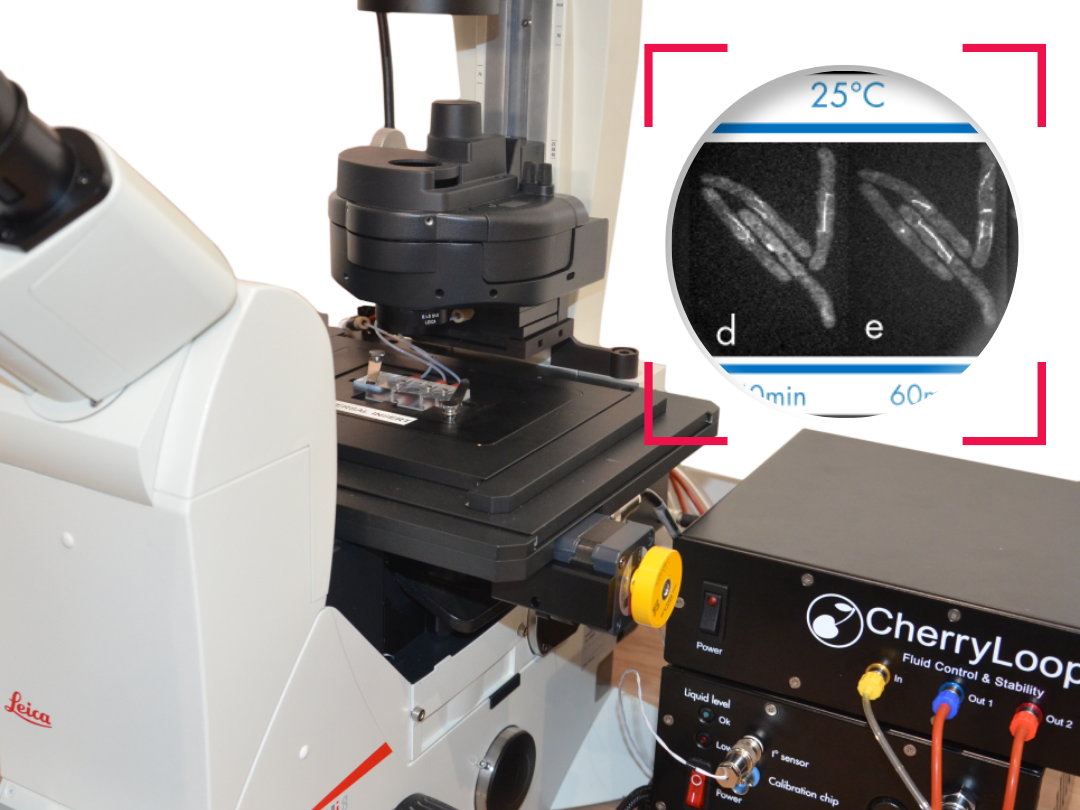
Ultra fast temperature shift device for in vitro experiments under microscopy
Wide-field fluorescence microscopy: zoomed at the atomic level
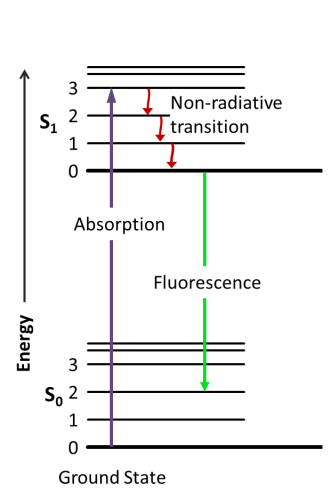
Fluorescence is the emission of electromagnetic radiation produced by the decay of an excited electron from the singlet state to its ground state (figure 1). The absorption of a photon with an energy of hѵ induces an orbital electron of a fluorophore to pass from the energetic level So to S1. S1 energy is equal to So + hѵ. Excited electrons in S1 typically relax by non-radiative transition before eventually ending up in a singlet state and decaying to the ground state by emitting electromagnetic radiation, typically visible light. While non-radiative phenomena (NRP) result in heat transfer to the nearby environment, the fluorescence emission generates a photon whit an energy that is equal to So + hѵ – NRPenergy. Thus the emitted photon in case of single-photon absorption has a longer wavelength (and thus less energy). This phenomenon, known as Stoke’s law, is rather important since as higher is the shift in wavelength, easier is to separate emission light from the excitation light by using filters able to exclude selected wavelengths (Fluo filters).
Fluorescence emission is much faster (10-8 seconds) than phosphorescence (minutes to hours) and disappears almost immediately once the excitation radiation is removed. For these reasons illumination and sample imaging should occur at the same time thus the necessity of applying filters to remove the band above a specific wavelength. The detection limit of an emitting sample depends on the ratio between the emission itself and the background noise. More the background is dark more the capability to observe fluorescence is high.
Since fluorescent emission is a statistically quite rare event depending on the fluorophore quantum yield, in a typical fluorescent microscope the light used to illuminate and excite the sample is several order of magnitude higher than the emitted fluorescence.
The need to strongly irradiate the sample has few drawbacks. Live specimens, especially when using shorter wavelengths to stimulate fluorescent emission, can suffer from photo-toxicity at high photon doses. The second aspect that comes from this high intensity irradiation is that the probability of secondary photochemical reactions increases significantly. Secondary photochemical reactions are the primary cause of decreasing emission among time. This phenomenon, called photobleaching, is a relevant issue when using some fluorescence microscopy techniques. Since the discovery of the photobleaching mechanisms scientist demonstrated that more than the intensity is the dose more implied in photobleaching. In other words a continuous milder irradiation might bring to more fluorophore loss of intensity than applying the same amount of photons in intense pulses. Among years several different microscopy configurations based on fluorescent microscopy have been developed. In the next paragraph we will list the most common fluorescent microscopy configurations.
Fluorescent microscopy techniques
Fluorescence is the core principle of several microscopy techniques developed in the last century. Techniques based on fluorescence microscopy have been applied to many different scientific and industrial fields; we will here focus on biological applications of fluorescence microscopy.
Wide-field fluorescence microscopy has opened many possibilities in life science especially considering the significant amount of molecules developed to “see” specific targets. By labeling specific cells structures or molecules scientists can visualize in real time changes in the distribution/conformation. Tagging cells according to the expression of specific surface ligands or the expression of selected genes is also possible and useful to monitor cells migrations, cell phenotyping etc. Cells genetically manipulated to express ionotropic channels sensitive to selected wavelengths allowed scientists to activate singles neurons (optogenetics) and map the functions of specific cells inside their neural circuit.
Three main approaches are used to properly label biological samples.
First approach uses natural or synthetic molecules that have a particular selectivity for specific molecules. Those molecules are often naturally fluorescent (i.e. Dapi, Draq5) or are chemically modified by binding a fluorophore (i.e. phalloidine).
Second approach is particularly used to visualize proteins of interest and is based on antibodies selectivity. In the simplest case an antibody against a specific protein is conjugated with a fluorophore and directly used to visualize the presence and distribution of a target protein inside or outside cells.
The third approach is based on genetically engineered cells that produce fluorescent proteins when specific genes are expressed.
Different factors must be considered when choosing the most convenient fluorescence microscopy technique. Those include: the desired spatial resolution; the dimension of the sample; the transparency of the sample; the concentration of a specific target; the presence of a strong autofluorescence background; the number of different targets; the nature (living/fixed) of the sample etc…
Hints on the characteristics of different fluorescent microscopy techniques with pros and cons can be found by clicking on the following links :
References
For more information please visit:
https://www.osapublishing.org/oe/fulltext.cfm?uri=oe-20-13-14534&id=238363
https://www.microscopyu.com/techniques/fluorescence/introduction-to-fluorescence-microscopy
http://zeiss-campus.magnet.fsu.edu/articles/superresolution/introduction.html
FAQ
Fluorescence is defined as a natural phenomenon. It begins with the absorption of light by a specific molecule known as a fluorophore. Almost immediately after this absorption, the molecule emits light. This emitted light possesses a longer wavelength than the absorbed light. This process has been widely exploited in many scientific fields, including microscopy. The technique of fluorescence microscopy is based on this capability. Samples are first labeled with specific fluorophores. The microscope system is then used to excite these fluorophores. Finally, the resulting emission of light from the sample is selectively collected by the instrument. The provided image, for example, shows actin filaments in an osteosarcoma cell line that were stained with phalloidin.
Fluorescence is the emission of electromagnetic radiation. This emission is produced when an excited electron decays from the singlet state (S1) back to its ground state (S0). First, a photon with specific energy (h\\𝜈) is absorbed. This absorption causes an orbital electron of the fluorophore to pass from the S0 energetic level to the S1 level. The energy of S1 is equal to S0 plus h\\𝜈. Electrons in the S1 state typically relax through non-radiative transitions. They eventually decay to the ground state by emitting electromagnetic radiation, which is usually visible light. The non-radiative phenomena (NRP) result in heat transfer. The fluorescence emission generates a photon with an energy equal to S0 \+ h\\𝜈 – NRPenergy. Therefore, the emitted photon has less energy and a longer wavelength. This is known as Stoke’s law.
A strong light source is needed to illuminate and excite the sample. This is because fluorescent emission is a statistically rare event. The light used is several orders of magnitude higher than the emitted fluorescence. This need for strong irradiation has drawbacks. Live specimens can suffer from photo-toxicity at high photon doses. This is especially true when shorter wavelengths are used. A second issue is photobleaching. High-intensity irradiation increases the probability of secondary photochemical reactions. These reactions are the main cause of emission decreasing over time. This loss of intensity is a known problem for some microscopy techniques. It has been demonstrated that the dose is more involved in photobleaching than the intensity alone. For instance, continuous milder irradiation might cause more fluorophore intensity loss than applying the same photon amount in intense pulses.
Three main approaches are used to properly label biological samples. The first approach uses natural or synthetic molecules. These molecules show a particular selectivity for specific targets. The molecules are often naturally fluorescent, such as Dapi, or they are chemically modified by binding a fluorophore, like phalloidine. The second approach is used to visualize proteins of interest. This method is based on antibody selectivity. In the simplest case, an antibody against a specific protein is conjugated with a fluorophore. This is then used to visualize the presence and distribution of the target protein. The third approach relies on genetically engineered cells. These cells are modified to produce fluorescent proteins when certain genes are expressed.