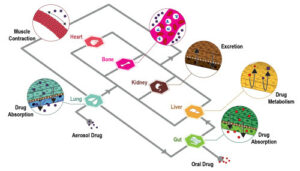
Introduction
Following our articles on how breast cancer organoids and breast‑cancer‑on‑chip platforms expand beyond conventional 2D and in vivo systems, we now focus on the decisive factor that underpins their translational value: comprehensive 3D breast cancer microenvironment modeling. Tumor progression, immune escape and therapy resistance arise from a dialogue between malignant epithelium and its niche, the tumor microenvironment, which houses stromal, immune and vascular partners within an extracellular matrix (ECM). Recreating this dialogue is essential to understanding drug resistance and pathophysiology. Here we outline the significance of each TME component in breast cancer modeling. We further discuss existing bioengineered breast cancer-on-chip and organoid co-culture models that incorporate TME to enhance tumor modeling for drug discovery and personalized medicine.
Learn more about our ready to use breast cancer organoid models.
Cells of the Breast Cancer microenvironments
The Breast cancer microenvironment is a complex network of different cell types that all plays a role in the disease agressivness and phenotypes.
Stromal Cells
- Fibroblasts and Cancer‑Associated Fibroblasts (CAF)
Fibroblasts are the principal stromal architects of the mammary gland: through paracrine FGF‑2/FGF‑9 signalling they proliferate, remodel collagen–fibronectin networks and guide ductal branching, providing the biomechanical and biochemical scaffold on which epithelial morphogenesis unfolds(1). During carcinogenesis, persistent inflammatory and growth‑factor cues co‑opt these otherwise homeostatic cells into cancer‑associated fibroblasts (CAF); an activated phenotype marked by α‑SMA, FAP and a secretory programme rich in matrix metalloproteinases (MMPs), cytokines and aligned ECM fibres.
The functional switch is of first‑order importance for breast‑tumor modelling. In microfluidic 3D co‑cultures, normal mammary fibroblasts already stimulate MDA‑MB‑231 cancer cell line invasion, but a fibronectin‑rich matrix containing CAFs doubles migration distance via MMP‑dependent tunnel formation as shown by Lugo-Citrón et al.(2). Organoid studies with autologous stromal cells reveal other mechanistic insight. For instance CAFs induce a basal, KRT14+ leader‑cell programme through TGF‑β/NOX4 signalling, thereby accelerating collective invasion(3). Beyond invasion, CAFs directly modulate therapeutic outcome: a HER2+ tumor‑on‑chip by Nguyen et al incorporating endothelial, immune and CAF compartments showed that CAFs suppress trastuzumab‑driven ADCC, antagonising antibody efficacy in real time(4).
Fibroblasts are also indispensable for modelling late‑stage stromal pathologies. A stroma‑rich MCF‑7/MRC‑5 spheroid platform recapitulates TGF‑β- or irradiation‑induced myofibroblast differentiation and excessive ECM deposition, enabling mechanistic dissection of radiation‑induced fibrosis in vitro(5). Collectively, these systems demonstrate that incorporating healthy fibroblasts and CAFs into 3D breast cancer microenvironments is critical for reproducing morphogenesis, invasion dynamics, drug resistance and fibrotic sequelae.
- Adipocyte
Recent research advances in adipose tissue are changing the status of this tissue, from an exclusive energy storage function to a more interlinked and complex role, particularly in fat rich tissue, such as the mammary gland. It is therefore not surprising that the 3D breast cancer microenvironment is containing mature adipocytes to supply lipids, adipokines and chronic inflammatory cues that accelerate breast‑tumor growth and therapy resistance. Incorporating these cells into 3D models captures critical metabolic and endocrine cross‑talk. Curious how to build physiologically relevant fat‑tumor platforms? Jump to our companion article on the role of adipose tissue in breast cancer.
Immune cells in breast cancer tumor microenvironment
As solid tumors evolve, they build biochemical (immunosuppressive stromal cells) and physical barriers (increased ECM deposition) that disable infiltrating leukocytes, allowing malignant cells to escape immune destruction. This reciprocal tug‑of‑war between cancer and immunity now sits at the heart of immuno‑oncology, where natural killer (NK) cells, cytotoxic T lymphocytes and tumor‑associated macrophages are dissected and engineered as therapeutic effectors. Their incorporation into 3D breast cancer models is particularly valuable for elucidating immune evasion mechanisms and evaluating novel immunotherapeutic strategies. While other myeloid and lymphoid cell types may be present in TME, we focus here on the role of NK, T cell and macrophages.
- Natural Killer and CAR-NK cells
Natural Killer (NK) cells patrol tissues for stress ligands and missing‑self cues, eliminating aberrant cells without prior sensitization. Clinically, adoptive infusion and CAR‑NK platforms are gaining traction for solid tumors, yet their efficacy stalls within the breast cancer microenvironment.
To investigate these mechanisms under controlled conditions, researchers have turned to advanced in vitro models that recreate the tumor–immune interface. Ayuso et al. recreated this milieu in a microfluidic tumor‑on‑a‑chip, showing that nutrient depletion, acidosis and hypoxia gradually exhaust NK cells (reduced granzyme expression, up‑regulated PD‑1/IDO‑1 and diminished cytotoxicity with checkpoint blockade only partially restoring function)(6). These findings highlight TME‑driven NK dysfunction and emphasize the need for physiologically relevant 3D assays when screening next‑generation NK immunotherapies.
- Lymphocyte T and CAR-T cells
Cytotoxic T lymphocytes (CTLs) recognize tumor neoantigens presented on MHC I and orchestrate adaptive anti‑cancer immunity, yet solid tumors frequently exclude or exhaust these cells via checkpoint signalling, metabolic competition and dense stroma. Engineering autologous T cells with chimeric antigen receptors (CAR‑T) bypasses HLA restriction and has transformed B‑cell leukaemia and lymphoma therapy, but translation to breast cancer is hindered by antigen heterogeneity, on‑target/off‑tumor risks and the physical–immunosuppressive barriers of the 3D breast cancer microenvironment. To better study these obstacles and test CAR‑T efficacy in a clinically relevant context, Maulana et al. used a perfused breast cancer‑on‑chip integrating patient‑derived organoids (PDOs) and endothelium to show CAR‑T cells that infiltrate, lyse PDOs and release cytokines while enabling real‑time monitoring of vascular extravasation and cytokine‑release‑syndrome surrogates, thus offering a patient‑specific efficacy–safety screen for next‑generation CAR‑T immunotherapies(7).
- Macrophages and Tumor-associated Macrophages
Macrophages are highly plastic phagocytes that, once educated by tumor‑derived chemokines, differentiate into tumor‑associated macrophages (TAMs) with an M2‑like, pro‑angiogenic programme. TAMs secrete VEGF, EGF and matrix‑remodelling proteases, guide endothelial sprouting and physically guide breast‑cancer cells toward intravasating vessels, correlating with high microvascular density and poor survival(8). Their inclusion in 3D breast‑cancer microenvironment models therefore provides an essential axis for probing immune‑escape circuits and stromal crosstalk. Raffo‑Romero et al. optimized three co‑culture formats combining human monocyte‑derived macrophages with patient tumoroids; live imaging and lipidomic profiling revealed bidirectional reprogramming and macrophage‑driven shifts in chemotherapy response, underscoring the predictive power of macrophage‑augmented tumoroids for therapeutic discovery(9).
Endothelial cell and Vascularization
Endothelial cells (ECs) are indispensable for advanced 3D breast cancer microenvironment models because they serve two complementary investigative purposes. First, a perfusable endothelial monolayer can be positioned as a selective barrier between the tumor milieu and a vascular channel, enabling quantitative studies of cancer‑cell intravasation/extravasation and immune‑cell recruitment to primary or secondary lesions. Second, tumors actively remodel their microenvironment by co‑opting macrophages and monocytes to secrete VEGF, EGF and MMPs, thereby stimulating angiogenic sprouting that nurtures growth and disseminates metastasis(8). Incorporating self‑assembled microvessels into 3D cultures is therefore essential for deciphering stromal pro‑angiogenic circuits and for screening anti‑vascular therapies.
A recent example by Ascheid et al. is a modular, fully human vascularised tumor spheroid platform comprising breast‑cancer cells, fibroblasts, ECs and THP‑1 macrophage analogues. Light‑sheet microscopy revealed intricate CD31⁺ pseudovessel networks; strikingly, removal of THP‑1 cells produced a ~40 % drop in relative vessel volume, without altering overall spheroid size, underscoring the macrophage dependency of tumor vascularisation(10). This proof‑of‑concept highlights how vascularized co‑cultures can rank TME‑targeted drugs and clarify leukocyte‑driven angiogenesis, guiding the rational design of anti‑angiogenic and immunomodulatory regimens.
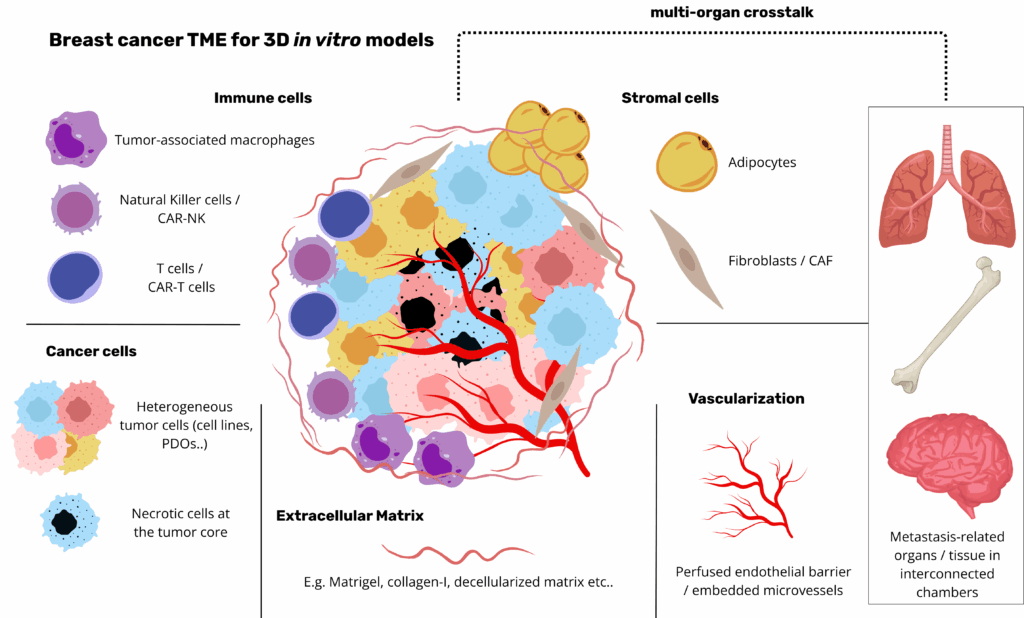
The ECM as scaffold for tumorigenesis
The breast extracellular matrix (ECM) comprises a laminin‑ and collagen IV‑rich basement membrane enveloping the epithelium and a fibrillar interstitial matrix dominated by collagen I/III, elastin, proteoglycans and glycosaminoglycans. Its biochemical composition is dynamically remodelled during tumorigenesis: downregulation of laminin‑III and the concomitant up‑regulation of laminin‑332 and MMP disrupt epithelial polarity, while matrix‑anchored growth factors sustain malignant signalling(11,12). Concurrently, increased expression of collagen elevates stiffness from ~200 Pa in healthy tissue to >1 kPa in invasive lesions, activating mechanotransduction pathways such as YAP/TAZ that drive proliferation and EMT(12). Cancer‑associated fibroblasts further reorganise collagen fibres into radially aligned tracks that guide cell migration and vascular infiltration. The ECM also acts as a molecular reservoir: bound TGF‑β, EGF and peptides orchestrate reciprocal tumor‑stromal crosstalk(11).
Matrix source critically influences model fidelity. Using a decellularized human mammary ECM hydrogel, Mollica et al. demonstrated that bioprinted mammary organoids reach larger, branching architectures and distinct Ki‑67/cytokeratin‑5 profiles compared with rat‑derived or Matrigel substrates, underscoring species‑specific cues(13). Nevertheless, Matrigel (a basement‑membrane extract rich in laminin and collagen IV) remains the most frequently used scaffold to mimic ECM for breast organoid culture, while collagen I, alginate and hybrid hydrogels provide tunable mechanics and bioactivity for disease modelling(11) (Buchholz et al., 2024). Capturing authentic ECM composition, stiffness, architecture and signalling is therefore paramount for predicting drug responses and unraveling breast‑cancer biology.
Bioengineering to Improve 3D Breast Cancer Microenvironment Modelling and Metastasis
To better recapitulate the TME and pathophysiological events, we now see emerging bioengineering strategies including bioprinting and breast cancer-on-chip.
Bioprinting (by extrusion or stereolithography methods) offer precise spatial reconstruction of cancer, stromal and endothelial cells within hydrogels, providing precise control over TME geometry(11). For instance, Langer et al. bioprinted breast cancer cells surrounded by fibroblasts and endothelial cells. They demonstrated that the tissue self-organized and deposited ECM. Notably their model revealed cancer subtypes differences in proliferation, signaling, ECM synthesis and invasion thus demonstrating the strengths of this method(14).
Some applications, however, such as metastasis studies, require multi-organ crosstalk and dynamic systems that are missing in static culture. Perfused organ‑on‑chip systems overcome these limitations by introducing flow, endothelial barriers and interconnected chambers.
Metastasis is the sequence of local invasion, intravasation, circulation, extravasation and colonization leading to secondary tumor sites in the body. The different subtypes of breast cancer have different organ tropism: luminal A/B tumors preferentially migrate to bone, HER2‑enriched tumors target liver and lung, while triple‑negative breast cancer (TNBC) exhibits a strong lung tropism(15). Cell‑cell and cell‑ECM dynamics highly influence this phenomenon. For instance, loss of laminin‑III with MMP up‑regulation disrupts the basement membrane and polarity, priming invasion.
Lab‑on‑chip platforms can model the different steps of the metastatic event. For instance Firatligil‑Yildirir et al. created parallel invasion/chemotaxis and extravasation chips lined with osteoblasts, marrow stroma and monocytes to quantify bone‑specific tropism of MDA‑MB‑231 clones(16). Crippa et al. engineered a perfusable vascularized breast‑to‑bone niche where neutrophils extravasate and modulate micrometastatic growth, highlighting immune contributions(17).
Conclusion
Breast cancer research is turning a decisive corner: by integrating epithelial, stromal, immune and vascular components within architecturally faithful, perfused 3D platforms, we can now interrogate the full tumor microenvironment in vitro. Such bioengineered models expose the cellular and matrix cues that drive progression, immune escape and metastasis, while offering high‑content platforms for drug screening and functional precision medicine. As biology and engineering converge, these next‑generation systems promise to shorten the path from mechanistic insight to personalized therapies.
Resources
- Sumbal J, Koledova Z. FGF signaling in mammary gland fibroblasts regulates multiple fibroblast functions and mammary epithelial morphogenesis. Development. 1 déc 2019
- Lugo-Cintrón KM, Gong MM, Ayuso JM, Tomko LA, Beebe DJ, Virumbrales-Muñoz M, et al. Breast Fibroblasts and ECM Components Modulate Breast Cancer Cell Migration through the Secretion of MMPs in a 3D Microfluidic Co-Culture Model. Cancers. 6 mai 2020;12(5):1173.
- Hanley CJ, Henriet E, Sirka OK, Thomas GJ, Ewald AJ. Tumor-Resident Stromal Cells Promote Breast Cancer Invasion through Regulation of the Basal Phenotype. Mol Cancer Res. 1 nov 2020;18(11):1615‑22.
- Nguyen M, De Ninno A, Mencattini A, Mermet-Meillon F, Fornabaio G, Evans SS, et al. Dissecting Effects of Anti-cancer Drugs and Cancer-Associated Fibroblasts by On-Chip Reconstitution of Immunocompetent Tumor Microenvironments. Cell Rep. déc 2018;25(13):3884-3893.e3.
- Yakavets I, Francois A, Benoit A, Merlin JL, Bezdetnaya L, Vogin G. Advanced co-culture 3D breast cancer model for investigation of fibrosis induced by external stimuli: optimization study. Sci Rep
- Ayuso JM, Rehman S, Virumbrales-Munoz M, McMinn PH, Geiger P, Fitzgerald C, et al. Microfluidic tumor-on-a-chip model to evaluate the role of tumor environmental stress on NK cell exhaustion. Sci Adv
- Maulana TI, Teufel C, Cipriano M, Roosz J, Lazarevski L, Van Den Hil FE, et al. Breast cancer-on-chip for patient-specific efficacy and safety testing of CAR-T cells. Cell Stem Cell. juill 2024;31(7):989-1002.e9.
- Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. Npj Breast Cancer
- Raffo-Romero A, Ziane-Chaouche L, Salomé-Desnoulez S, Hajjaji N, Fournier I, Salzet M, et al. A co-culture system of macrophages with breast cancer tumoroids to study cell interactions and therapeutic responses. Cell Rep Methods. juin 2024;4(6):100792.
- Ascheid D, Baumann M, Pinnecker J, Friedrich M, Szi-Marton D, Medved C, et al. A vascularized breast cancer spheroid platform for the ranked evaluation of tumor microenvironment-targeted drugs by light sheet fluorescence microscopy. Nat Commun
- Buchholz MB, Scheerman DI, Levato R, Wehrens EJ, Rios AC. Human breast tissue engineering in health and disease. EMBO Mol Med. 23 août 2024;16(10):2299‑321.
- Bahcecioglu G, Basara G, Ellis BW, Ren X, Zorlutuna P. Breast cancer models: Engineering the tumor microenvironment. Acta Biomater. avr 2020;106:1‑21.
- Mollica PA, Booth-Creech EN, Reid JA, Zamponi M, Sullivan SM, Palmer XL, et al. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. sept 2019;95:201‑13.
- Langer EM, Allen-Petersen BL, King SM, Kendsersky ND, Turnidge MA, Kuziel GM, et al. Modeling Tumor Phenotypes In Vitro with Three-Dimensional Bioprinting. Cell Rep. janv 2019;26(3):608-623.e6.
- Firatligil-Yildirir B, Yalcin-Ozuysal O, Nonappa. Recent advances in lab-on-a-chip systems for breast cancer metastasis research. Nanoscale Adv. 2023;5(9):2375‑93.
- Firatligil-Yildirir B, Bati-Ayaz G, Pesen-Okvur D, Yalcin-Ozuysal O. Invasion/chemotaxis- and extravasation-chip models for breast cancer bone metastasis.
- Crippa M, Talò G, Lamouline A, Bolis S, Arrigoni C, Bersini S, et al. A microfluidic model of human vascularized breast cancer metastasis to bone for the study of neutrophil-cancer cell interactions. Mater Today Bio. déc 2022;17:100460.
FAQ
Tumour progression, immune escape, and therapy resistance are understood to arise from a dialogue. This dialogue occurs between malignant epithelium and its niche, which is the tumour microenvironment, or TME. This niche is complex. It houses stromal, immune, and vascular partners. These different components are all held within an extracellular matrix, known as the ECM. The recreation of this dialogue is believed to be required. This is needed to properly understand drug resistance. It also helps in understanding the pathophysiology of the disease. Models that can comprehensively show this 3D breast cancer microenvironment are being developed. These platforms are used in drug discovery and personalized medicine.
Fibroblasts are the main stromal architects of the mammary gland. During carcinogenesis, they are co-opted by persistent inflammatory and growth-factor cues. This turns them into cancer-associated fibroblasts, or CAFs. This is an activated phenotype. It is marked by α-SMA and FAP. CAFs have a secretory programme. This programme is rich in matrix metalloproteinases (MMPs) and aligned ECM fibres. In microfluidic 3D co-cultures, CAFs were shown to double the migration distance of a cancer cell line. This was an MMP-dependent process. Other organoid studies showed CAFs induce a basal leader-cell programme. This acceleration of collective invasion occurs through TGF-β/NOX4 signalling. CAFs can also modulate therapy. A HER2+ tumour-on-chip showed CAFs suppress trastuzumab-driven ADCC, antagonising antibody efficacy.
Mature adipocytes are part of the 3D breast cancer microenvironment. Lipids and adipokines are supplied by these cells. Chronic inflammatory cues are also supplied. These factors are understood to accelerate breast-tumour growth. They also promote therapy resistance. The incorporation of these cells into 3D models is done. This allows for the capture of metabolic and endocrine cross-talk. Adipose tissue’s function is complex. It has changed from just an energy storage function. It is now seen as having a more interlinked role, especially in fat-rich tissues like the mammary gland.
Solid tumours evolve as they grow. Biochemical barriers are built. These include immunosuppressive stromal cells. Physical barriers are also built. An example is increased ECM deposition. These barriers work to disable infiltrating leukocytes. Malignant cells are then allowed to escape immune destruction. This reciprocal interaction between cancer and immunity is a central topic in immuno-oncology. Natural killer (NK) cells, cytotoxic T lymphocytes (T-cells), and tumor-associated macrophages (TAMs) are studied. These cells are being engineered as therapeutic effectors. Their incorporation into 3D breast cancer models is considered useful. It is done for elucidating immune evasion mechanisms and evaluating new immunotherapeutic strategies.
Natural Killer (NK) cells normally patrol tissues. They look for stress ligands and missing-self cues. Aberrant cells are eliminated by them without prior sensitization. Their efficacy, however, is known to stall within the breast cancer microenvironment. Advanced in vitro models are used to investigate these mechanisms. A microfluidic tumour-on-a-chip was used to recreate this milieu. It was shown that NK cells become exhausted over time. This exhaustion is caused by nutrient depletion, acidosis, and hypoxia. The exhausted state was characterized by reduced granzyme expression. Upregulation of PD-1 and IDO-1 was also seen. Cytotoxicity was diminished. These findings show TME-driven NK dysfunction.
Translation of CAR-T therapy to breast cancer is difficult. This is hindered by antigen heterogeneity and the physical barriers of the 3D breast cancer microenvironment. A perfused breast cancer-on-chip platform was used to study these obstacles. This system integrated patient-derived organoids (PDOs) and an endothelium. CAR-T cells were shown to infiltrate the system. They were also shown to lyse the PDOs and release cytokines. This setup allowed for real-time monitoring of vascular extravasation. Surrogates for cytokine-release-syndrome were also monitored. The platform is offered as a patient-specific efficacy and safety screen. It is used for testing next-generation CAR-T immunotherapies.
Macrophages are known as highly plastic phagocytes. They can be educated by tumour-derived chemokines. Once educated, they differentiate into tumour-associated macrophages (TAMs). TAMs typically have an M2-like, pro-angiogenic programme. They secrete substances such as VEGF and EGF. Matrix-remodelling proteases are also secreted. Endothelial sprouting is guided by them. They also physically guide breast-cancer cells toward intravasating vessels. A correlation is seen with high microvascular density and poor survival. Their inclusion in 3D models provides an axis for probing immune-escape circuits. Co-culture formats with patient tumoroids have revealed bidirectional reprogramming. Macrophage-driven shifts in chemotherapy response were also shown.
Endothelial cells (ECs) are considered indispensable for advanced 3D breast cancer microenvironment models. They serve two complementary investigative purposes. First, a perfusable endothelial monolayer can be positioned as a selective barrier. This barrier is placed between the tumour milieu and a vascular channel. This setup allows for quantitative studies of cancer-cell intravasation and extravasation. The recruitment of immune-cells can also be studied. Second, tumours actively remodel their microenvironment. Macrophages are co-opted to secrete VEGF, which stimulates angiogenic sprouting. Incorporating self-assembled microvessels into 3D cultures is therefore done. This is needed for deciphering stromal pro-angiogenic circuits. It is also used for screening anti-vascular therapies.
The breast extracellular matrix (ECM) has a biochemical composition. This composition is dynamically remodelled during tumorigenesis. A downregulation of laminin-III occurs. At the same time, an upregulation of laminin-332 and MMPs happens. This disrupts epithelial polarity. Concurrently, an increased expression of collagen elevates stiffness. Stiffness can change from approximately 200 Pa in healthy tissue to over 1 kPa in invasive lesions. This change activates mechanotransduction pathways, such as YAP/TAZ. These pathways are associated with proliferation and EMT. Cancer-associated fibroblasts also reorganise collagen fibres. They are formed into radially aligned tracks. These tracks guide cell migration and vascular infiltration. The ECM also acts as a molecular reservoir for growth factors.
Bioprinting offers precise spatial reconstruction of cancer, stromal, and endothelial cells within hydrogels. This provides exact control over the TME geometry. Studies have shown that bioprinted tissue can self-organize and deposit ECM. Cancer subtype differences in proliferation and invasion were revealed with this method. Some applications, like metastasis studies, require multi-organ crosstalk and dynamic systems. These features are missing in static cultures. Perfused organ-on-chip systems are used to overcome these limitations. They introduce flow, endothelial barriers, and interconnected chambers. These platforms can model different steps of the metastatic event. For instance, chips have been created to quantify bone-specific tropism. A perfusable vascularized breast-to-bone niche has also been engineered.