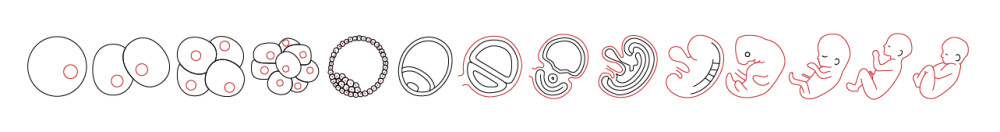
Embryogenesis in a dish
Thanks to the last discoveries, a new path has been opened in the human embryo culture and embryonic stem cell culture. Until now, growing in vitro mouse or human embryos were technically limited to cultures that only reached blastocyst stage (5 or 6 days development). The main events in the embryogenesis, especially the ones related to the developmental disorders, occurs just right after those days (1), highlighting the need of having access to older stage embryos.
Since 2016, it is possible to culture human embryos up to 13 days because of technical improvements. This is allowing us to observe many of the key structures and events that will later support the growth of the embryo. Indeed, after 14 cell cycles, the embryo is preparing itself to undergo the gastrulation, which is the main organizational event that generates the basic body plan and provides a base to build all the tissues (2-3).
The ability of embryonic stem cells to divided and be renewed make them a good and easily handling experimental model. Furthermore, and by definition, they can become any types of cells after going through many key stages of development. While the in vivo embryogenesis is well spatially organized, in vitro differentiation of embryonic stem cells is not. The possibility for researchers to engineer the microenvironment to make it suitable for the three-dimensional tissue differentiation is a key advance. It is now possible to provide devices and structures that closely mimic the shape of a differentiating embryo (4-5). Going in that way, there are some recommendations from Harrison and colleagues (6) that could led us to the final goal of mimicking human embryogenesis in a dish. One of the main drawback is that the supply of human embryos is limited and very few of these embryos reach advanced developmental stages. Because extra-embryonic tissues (placenta or yolk sac) provide, in addition to nutrients, signals that drive the differentiation of pluripotent cells in a regulated spatial and temporal domain, combining embryonic stem cells with trophoblast stem cells might be a solution. Indeed, the combination of these two types of cells in a gel containing extracellular matrix molecules produces promising results in mouse.
Even if these replicas of mouse embryos were not perfect they represent a first step on the race to make embryogenesis on a dish. The key now is to understand how refining the culture environment (spatial, chemical and physical) for the embryos to develop. To do so, a multidisciplinary research will be required: e.g biologist will need help from biochemist and engineer to develop a scaffold for the embryos to growth.
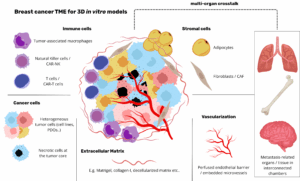
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
References
- Pera M. Embryogenesis in a dish. Science
- Deglincerti et al., Nature 533, 251 (2016).
- N. Shahbazi et al., Nat. Cell Biol. 18, 700 (2016).
- Etoc et al., Dev. Cell 39, 302 (2016).
- Shao et al., Nat. Mater. 16, 419 (2016).
- E.Harrison, B. Sozen, N. Christodoulou, C. Kyprianou, M. Zernicka-Goetz,Science 356, eaal1810 (2017)
FAQ
Until recently, growing mouse or human embryos in vitro was technically restricted. Cultures were only able to reach the blastocyst stage of development. This stage corresponds to approximately five or six days. This limitation was a significant problem for research into human development. Many of the important events in embryogenesis, especially those related to developmental disorders, occur just after this period. The inability to sustain cultures beyond this point created a gap in knowledge. Therefore, a need to access and study embryos at older stages was noted by the scientific community. The inability to observe these later processes restricted the understanding of important organizational phases.
A new direction has been opened in human embryo culture due to recent findings. Technical developments made since 2016 have made it possible to culture human embryos for up to 13 days. This is an extension from the previous limit of the blastocyst stage. This extended culture period allows researchers to observe many of the structures and events that support the later growth of the embryo. Specifically, after 14 cell cycles, the embryo can be seen preparing for gastrulation. Gastrulation is described as the principal organizational event. It is responsible for creating the basic body plan and provides a base for building all tissues.
Embryonic stem cells are considered a useful and easily handled experimental model. This is because of their ability to divide and be renewed. By definition, they also have the capacity to become any type of cell. This requires them to go through many stages of development. A central difficulty exists in using them for differentiation in vitro. While embryogenesis in vivo is well-organized in its spatial arrangement, the in vitro differentiation of these stem cells is not. This lack of organization is a problem. A development has been made in the possibility for researchers to engineer the microenvironment. It is now possible to supply devices and structures that make the environment suitable for three-dimensional tissue differentiation. These structures are designed to closely mimic the shape of a differentiating embryo.
One of the central drawbacks to this research is the limited availability of human embryos. Very few of these embryos are able to reach later developmental stages. This limits the source material for study. A possible solution is being explored to address this and to better mimic the natural environment. In the body, extra-embryonic tissues like the placenta or yolk sac furnish nutrients. They also send signals that direct the differentiation of pluripotent cells. This guidance occurs in a regulated spatial and temporal domain. To replicate this, researchers are combining embryonic stem cells with trophoblast stem cells. This combination has been tested in a gel that contains extracellular matrix molecules. The approach has produced encouraging results in mouse models, though these replicas were not perfect.