Introduction
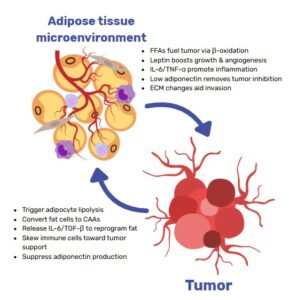
In postmenopausal women, fat mass and adipose-tissue mass (see Human Breast Adipocytes) are closely linked to obesity and breast cancer. Adipocytes have the ability to modulate the tumor microenvironment by secreting energy nutrients, which increases the risk of breast cancer incidence, proliferation, and metastasis.
Physical and emotional repercussions are significantly linked to cancer incidence and growth, in addition to other obesity-related issues. As a result, it’s critical to comprehend how adipose tissues and adipocytes can influence breast cancer risk and mortality.
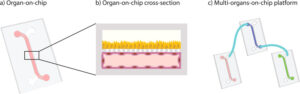
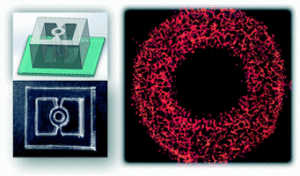
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
Background/aim: Recent research highlights the role of cancer-associated adipocytes (CAA) in promoting breast cancer cell migration, invasion and resistance to therapy. This study aimed at identifying cellular proteins differentially regulated in breast cancer cells co-cultured with CAA.
Materials and methods: Adipocytes isolated from human breast adipose tissue were co-cultured with hormone receptor-positive (MCF-7) or -negative (MDA-MB-231) breast cancer cells using a transwell co-culture system. Proteomes of co-cultured and control breast cancer cells were compared quantitatively using iTRAQ labelling and tandem mass spectrometry, and the results were validated by western blotting.
Results: A total of 1,126 and 1,218 proteins were identified in MCF-7 and MDA-MB-231 cells, respectively. Among these, 85 (MCF-7) and 63 (MDA-MB-231) had an average fold change >1.5 following co-culture. Pathway analysis revealed that CAA-induced enrichment of proteins involved in metabolism, the ubiquitin proteasome, and purine synthesis.
Conclusion: This study provides a proteomic platform for investigating the paracrine role of CAA in promoting breast cancer cell metastasis and resistance to therapy.
References
Lee Isla Crake R, Phillips E, Kleffmann T, Currie MJ. Co-culture With Human Breast Adipocytes Differentially Regulates Protein Abundance in Breast Cancer Cells. Cancer Genomics Proteomics. 2019 Sep-Oct;16(5):319-332. doi: 10.21873/cgp.20137. PMID: 31467226; PMCID: PMC6727076.