Re-Engineering the Liver Microenvironment
In the first article of this series, we discussed the limitations of conventional models of the liver and how 3D cell culture systems such as organoids and spheroids represent a significant step forward in preserving hepatocyte function and enabling more predictive studies of drug metabolism and toxicity. If you are interested in this background you can access the article here. In this second part, we focus on liver-on-chip platforms, where advances in microengineering and biosensing converge with cell biology to further increase physiological relevance.
Learn more about our ready to use human liver organoid models.
Emergence of Organ-on-Chip Technology
The concept of organ-on-a-chip (OoC) technology was formulated in the early 2000s from the convergence of microfabrication, microfluidics, and cell culture. The objective was to move beyond static three-dimensional cultures and design continuously perfused microenvironments capable of replicating organ-level functions in vitro. This framework was shaped by the seminal work of Ingber and colleagues at Harvard’s Wyss Institute, who defined OoCs not as culture devices but as systems intended to reproduce key physiological processes of intact organs.
Their landmark publication in 2010 demonstrated the feasibility of this approach with the lung-on-a-chip(1), which successfully recreated the alveolar–capillary interface and incorporated cyclic stretching to mimic breathing. The device not only supported barrier function but also modeled immune responses to bacterial infection. This achievement provided a clear demonstration that organ-on-chip systems could capture not just cellular composition but also dynamic mechanical and biochemical functions, catalyzing efforts to extend the approach to the liver.
Early Liver-on-Chip Architectures
Early liver-on-chip models aimed to address the rapid loss of function observed in two-dimensional hepatocyte cultures. Microfabrication strategies, such as micropatterning hepatocytes with endothelial or stromal cells, enhanced intercellular communication and substantially increased cytochrome P450 (CYP450) activity compared to random co-cultures(2). Progressively, microfluidic platforms were introduced, often fabricated from polydimethylsiloxane (PDMS)(3), with parallel channels separated by porous membranes. These designs enabled the co-culture of hepatocytes and liver sinusoidal endothelial cells in a configuration that reflected the hepatic sinusoid. Other designs adopted hexagonal chamber geometries to approximate the lobular organization of the liver and to generate physiologically relevant gradients of oxygen and nutrients(4). Collectively, these advances extended hepatocyte viability and functionality, establishing a foundation for more sophisticated liver-on-chip systems.
Evolution of Cellular Sources
The predictive value of a liver-on-chip platform is determined not only by its architecture but also by the cellular sources employed.
- Immortalized cell lines (e.g., HepG2, Huh-7, HepaRG) were widely used in early research phases due to their robustness and scalability. However, they exhibit immature phenotypes and limited metabolic capacity, making them suboptimal for detailed pharmacokinetic or toxicology studies.
- Primary human hepatocytes (PHHs) remain the benchmark for physiological relevance. In conventional culture, PHHs lose function rapidly, but within liver-on-chip systems, where they benefit from perfusion, three-dimensional context, and co-culture with non-parenchymal cells, they can sustain albumin and urea secretion, transporter activity, and stable CYP450 expression for weeks. Notably, Jang et al. demonstrated that species-specific liver chips containing PHHs, rat hepatocytes, or dog hepatocytes could replicate known interspecies differences in drug-induced liver injury, highlighting their translational value(5).
Human pluripotent stem cell–derived hepatocyte-like cells (HLCs) represent an emerging frontier. Induced pluripotent stem cells (iPSCs) provide an effectively unlimited and genetically diverse source of hepatocytes. Although HLCs often display fetal-like characteristics, advances in differentiation protocols and the use of dynamic microenvironments are improving maturation. Importantly, iPSC-derived hepatocytes enable patient-specific models, creating opportunities to investigate idiosyncratic responses and genetic disorders in a controlled in vitro setting.
Engineering Principles of Physiological Relevance
The ability of liver-on-chip systems to recapitulate organ-level functions arises from several interrelated engineering features:
- Microfluidics and perfusion
- Three-dimensional architecture
- Multicellular co-culture
- Integrated biosensors
Microfluidics and perfusion
The defining feature of all organ-on-a-chip systems is the use of microfluidics—the science and technology of manipulating fluids in channels with dimensions of tens to hundreds of micrometers. In LoC, microfluidic channels serve as an artificial vascular network, enabling the continuous perfusion of culture medium to simulate the constant flow of blood through the liver sinusoids.
This dynamic flow is fundamentally important for several reasons. First, it ensures a steady supply of oxygen and nutrients to the cells while simultaneously removing metabolic waste products, preventing the nutrient depletion and toxic accumulation that plague static cultures. This is critical for maintaining the health and viability of high-density liver cell cultures over long periods. Second, perfusion allows for precise control over the cellular microenvironment, enabling researchers to introduce drugs, hormones, and signaling molecules at stable, physiologically relevant concentrations.
A crucial consequence of this continuous, unidirectional flow is the natural establishment of chemical gradients along the length of the microchannel. As medium flows past the cells, oxygen and nutrients are consumed, and metabolites are released, creating a gradient from the inlet to the outlet. This process mimics the zonation that occurs in the native liver lobule, where hepatocytes in “zone 1” (periportal, near the blood inlet) are exposed to highly oxygenated, nutrient-rich blood and have different metabolic functions (e.g., gluconeogenesis) than hepatocytes in “zone 3” (pericentral, near the blood outlet), which see lower oxygen levels and specialize in functions like detoxification and lipogenesis. The ability of LoC to induce this metabolic zonation in vitro represents a major step toward recapitulating the liver’s complex functional organization.
Finally, the flow of fluid exerts a physical force on the cells known as fluid shear stress. This is a critical biomechanical cue that is completely absent in static cultures. While excessive shear stress can damage hepatocytes, which are shielded from direct high-velocity flow in the body, controlled, low levels of shear (typically below 5 dyn/cm²) have been shown to be beneficial, helping to maintain proper cell morphology and function(6). Researchers often use computational fluid dynamics (CFD) simulations to precisely model and engineer the flow profiles within a chip to achieve physiologically appropriate shear stress levels
Three-dimensional architecture
The transition from flat 2D surfaces to a three-dimensional (3D) culture environment is another fundamental requirement for achieving physiological relevance. In the native liver, hepatocytes are organized into complex 3D structures where they are in constant communication with neighboring cells and the surrounding extracellular matrix (ECM). This 3D context is essential for maintaining hepatocyte polarity (the distinct apical and basolateral surfaces required for bile secretion and nutrient uptake), cuboidal morphology, and high-level metabolic function(7).
Many LoC designs utilize biocompatible hydrogels, such as collagen or Matrigel, as scaffolds to provide structural support for the cells and to mimic the native ECM. Cells are often encapsulated within these gels, which promotes the self-assembly of 3D liver microtissues. An alternative and widely used approach is the formation of 3D cell aggregates, known as spheroids, or more complex, self-organizing structures called organoids. These 3D structures can be formed prior to being loaded into a chip or can be encouraged to form in situ. Integrating these spheroids or organoids into a microfluidic device combines the benefits of a complex 3D architecture with the advantages of dynamic perfusion.
While these methods are effective, there is a design tension between achieving perfect biomimicry and ensuring usability and high throughput. Highly complex chip designs that attempt to precisely replicate the liver’s lobular architecture can be difficult to fabricate, operate reliably, and scale for the needs of pharmaceutical drug screening. Consequently, many commercial platforms opt for simplified yet effective designs that balance physiological relevance with practicality.
Multicellular co-culture
The liver is not a monoculture of hepatocytes. It is a complex, multicellular organ where hepatocytes (parenchymal cells) work in close concert with a variety of non-parenchymal cells (NPCs). The most important NPCs include liver sinusoidal endothelial cells (LSECs), which line the sinusoids and form a porous barrier between the blood and the hepatocytes; Kupffer cells, the resident macrophages of the liver that play a key role in immunity and inflammation; and hepatic stellate cells (HSCs), which store vitamin A and are the primary source of scar tissue during fibrosis. These NPCs are critical for normal liver function and are central to the pathogenesis of most liver diseases.
Therefore, to improve physiological relevance, a LoC must be a multicellular system. Co-culturing hepatocytes with these NPCs is essential for stabilizing hepatocyte function through paracrine signaling and direct cell-cell contacts. A common and effective LoC design, among others, for achieving this is the dual-channel system, where hepatocytes are cultured in one channel and NPCs, particularly LSECs, are cultured on the opposite side of a porous membrane in an adjacent channel. This architecture directly recreates the fundamental tissue-tissue interface of the liver sinusoid, with a vascular compartment and a parenchymal compartment.
The inclusion of specific NPCs is tailored to the research question. For studies on inflammation or immune-mediated DILI, the inclusion of Kupffer cells is indispensable. For modeling liver fibrosis, the presence of hepatic stellate cells is non-negotiable, as their activation is the central event in the fibrotic process. This ability to build a liver model with a defined and relevant cellular composition is a key advantage of LoC technology.
Integrated biosensors for real-time, non-invasive monitoring
A significant limitation of traditional cell culture is its reliance on end-point assays, which are often destructive and provide only a single snapshot in time. An emerging and powerful feature of LoC technology is the ability to integrate miniaturized biosensors directly into the chip platform. This enables continuous, real-time, and non-invasive monitoring of the health and metabolic activity of the liver tissue.
While still a developing area, researchers have successfully integrated various types of sensors into LoC devices and they are now being incorporated in commercially available OoC models. These include electrodes to measure the transepithelial/transendothelial electrical resistance (TEER), which provides a quantitative measure of the integrity of cellular barriers like the sinusoidal endothelium. Other sensors can monitor the oxygen consumption rate (OCR), a key indicator of metabolic activity, or track the concentrations of crucial metabolites like glucose and lactate in the culture medium.
This capability for real-time monitoring is invaluable for pharmacokinetic and toxicological studies. It allows researchers to observe the full dynamic response of the liver tissue to a drug exposure over time, capturing the precise onset of toxicity, transient metabolic effects, or mechanisms of cellular adaptation that would be completely missed by a single end-point measurement. This provides a much richer and more informative dataset for making critical decisions in drug development.
Applications and Case Studies in Drug Discovery
For pharmaceutical research, liver-on-chip technology offers a platform that combines the strengths of 3D cell culture with additional layers of physiological realism provided by flow, multicellular organization, and integrated sensing. These systems support chronic dosing, more predictive detection of DILI, and mechanistic understanding of drug metabolism and drug–drug interactions. Positioned within the broader landscape of New Approach Methodologies (NAMs), liver-on-chip systems hold the potential to reduce reliance on animal testing and to provide more translatable human-relevant data early in the drug development process.
Use Cases of Liver-on-Chip Systems
The translational impact of liver-on-chip (LoC) technology is becoming increasingly clear. Building on the conceptual advances in 3D cell culture and organoids, these microphysiological systems now provide human-relevant data across the drug discovery pipeline. By maintaining metabolic competence, enabling long-term culture, and incorporating real-time monitoring, LoC platforms address critical bottlenecks in predicting pharmacokinetics, drug-induced liver injury, drug–drug interactions, and complex disease mechanisms.
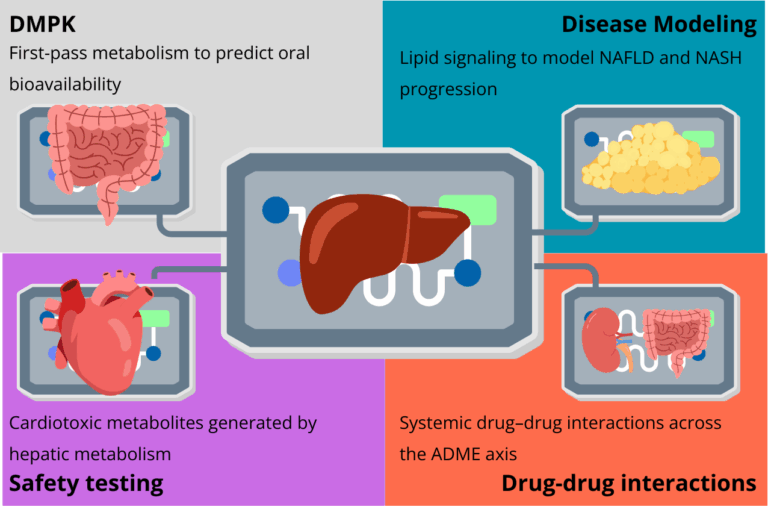
Drug Metabolism and Pharmacokinetics (DMPK)
Characterizing drug metabolism and pharmacokinetics (DMPK) is fundamental to determining whether a candidate can become a safe and effective therapy. Traditional 2D hepatocyte models fail to sustain enzyme activity long enough to assess low-clearance compounds, leading to gaps in in vitro–in vivo extrapolation (IVIVE).
Liver-on-chip systems overcome this limitation by preserving high levels of cytochrome P450 (CYP450) and conjugating enzyme activity for weeks. This allows researchers to measure clearance rates, identify metabolites, and quantify the fraction metabolized (fm) through specific pathways with greater accuracy(8,9). Both Phase I (oxidation, reduction, hydrolysis) and Phase II (glucuronidation, sulfation) reactions can be studied under stable conditions, directly informing IVIVE models and reducing uncertainty in human pharmacokinetic predictions.
Another strength of LoC technology is modularity. By linking a gut-on-chip with a liver-on-chip, researchers can recreate first-pass metabolism, which strongly influences oral bioavailability(10). This type of multi-organ configuration provides insights that static organoid or spheroid models cannot capture, offering a more physiologically relevant basis for drug discovery and development.
Safety and Toxicity Assessment: Advancing DILI Prediction
One of the most mature and impactful applications of LoC is in safety pharmacology, particularly for drug-induced liver injury (DILI). Traditional preclinical models, including animal testing, have repeatedly failed to predict hepatotoxicity in humans, contributing to costly late-stage attrition.
A landmark validation study by Ewart et al, conducted with multiple pharmaceutical partners under the IQ MPS Consortium, tested 870 Liver-Chips with 27 compounds of known clinical outcomes. The platform correctly identified hepatotoxic drugs with 87% sensitivity and 100% specificity, far outperforming animal models and static 3D cultures. Importantly, these were compounds previously classified as safe in animals but later withdrawn from the market for causing severe liver injury in patients. Integrating Liver-Chips into preclinical pipelines could therefore reduce hepatotoxic compounds entering trials by nearly eightfold, with estimated savings of over $3 billion annually across the industry(11).
LoC platforms also allow mechanistic dissection of liver injury. Specific assays can distinguish apoptosis, steatosis, or cholestasis. By incorporating Kupffer cells and other immune components, LoCs can model immune-mediated DILI. Moreover, cross-species chips (human, rat, dog) enable translational comparisons, helping determine whether animal toxicity findings are relevant for humans, thereby refining the use of alternatives to animal testing(5).
Investigating Drug–Drug Interactions (DDIs)
Drug-Drug Interactions (DDIs) occur when one drug alters the ADME properties of a co-administered drug, potentially leading to reduced efficacy or increased toxicity. The liver, as the body’s metabolic hub, is the primary site of these interactions, which are often mediated by the induction or inhibition of CYP enzymes. With their stable and long-term metabolic competence, LoC systems provide an ideal platform for studying these complex metabolic DDIs.
However, the true power of organ-on-a-chip technology for DDI studies is realized in multi-organ systems. The toxicity of a drug or its metabolites is often a systemic property that arises from the dynamic interplay between different organs. Multi-organ-on-a-chip platforms are the first in vitro tool capable of capturing these emergent biological phenomena. By connecting a liver-chip with other organ models, such as a kidney-chip or a heart-chip, researchers can investigate how the hepatic metabolism of a drug produces metabolites that may be toxic to another organ. For example, studies have demonstrated that a parent drug may be non-toxic to cardiac cells in isolation, but becomes highly cardiotoxic after it is metabolized by the liver component in a connected multi-organ system. Conversely, the liver might detoxify a compound that is otherwise harmful to another organ. These platforms allow for the investigation of off-target toxicity and provide a more holistic, system-level view of a drug’s safety profile, which is impossible to achieve in single-organ models(12).
Advanced Disease Modeling
In addition to their applications in pharmacology and toxicology, LoC platforms are becoming increasingly valuable tools for studying the pathophysiology of human liver diseases. By recreating the key cellular and microenvironmental features of a diseased liver, these models provide a human-relevant platform for investigating disease mechanisms and screening for new therapies.
Metabolic Disorders: NAFLD and NASH
Nonalcoholic Fatty Liver Disease (NAFLD) and its progressive, inflammatory form, Nonalcoholic Steatohepatitis (NASH), have become a global health crisis. LoC models have proven highly effective at replicating the key pathological features of these diseases. The initial stage, steatosis (fat accumulation), is typically induced by perfusing the liver cells with a medium supplemented with high concentrations of free fatty acids, such as oleic and palmitic acid(13).
To model the progression to NASH, which involves inflammation and fibrosis, researchers introduce pro-inflammatory stimuli like lipopolysaccharide (LPS) or tumor necrosis factor-alpha (TNF-α) and pro-fibrotic factors like transforming growth factor-beta (TGF-β). The inclusion of Kupffer cells and hepatic stellate cells in the co-culture is critical for this, as the activation of these cells drives the inflammatory and fibrotic responses, respectively. These “NASH-on-a-chip” models exhibit key disease phenotypes, including hepatocyte injury, lipid accumulation, inflammation, and collagen deposition, providing a robust platform for testing the efficacy of anti-NAFLD and anti-fibrotic drug candidates(14,15).
Importantly, coupling liver chips with adipose tissue modules has expanded these models beyond hepatocellular events to capture the systemic metabolic dysfunction now recognized as central to Metabolic Associated Fatty Liver Disease (MAFLD). Adipose tissue is not only a fat storage depot but also an active endocrine organ secreting adipokines, cytokines, and free fatty acids that strongly influence hepatic metabolism. In multi-organ liver–adipose systems, chronic exposure of hepatocytes to adipose-derived lipids and pro-inflammatory mediators reproduces the “two-hit” or “multiple-hit” hypotheses of MAFLD/NASH pathogenesis: steatosis driven by lipid overload, followed by inflammation and fibrogenesis triggered by adipose-derived signals.
A notable example is the adipose–liver chip developed by Slaughter et al. (2021), which reproduced hallmarks of NAFLD progression and was used to evaluate the limited efficacy of metformin under physiologically relevant conditions. This study highlighted how inter-organ crosstalk, absent in isolated liver models, is essential for capturing the complexity of metabolic liver disease and for testing therapeutic responses in a human-relevant context.
Beyond NASH, liver–adipose chips are emerging as powerful tools to investigate the interplay between obesity, type 2 diabetes, and dyslipidemia, conditions where ectopic lipid deposition and adipokine imbalance directly impair hepatic function. By recapitulating the bidirectional signaling between fat and liver, these platforms offer a uniquely human-relevant approach to study metabolic syndrome at the organ-to-organ level and accelerate the development of therapies aimed at restoring metabolic flexibility.
Viral Hepatitis
Research into Hepatitis B virus (HBV) has been historically constrained by its host specificity, as it infects only humans and chimpanzees. Conventional hepatocyte cultures are too short-lived to sustain the full viral life cycle. Liver-on-chip systems, by contrast, maintain hepatocyte function for over 40 days, supporting HBV infection, replication, and virion release. This enables detailed study of host–pathogen interactions and immune evasion strategies, as well as testing of novel antiviral candidates. For viral hepatitis, where relevant animal models are scarce, LoC technology is not just an alternative but an indispensable research tool(16,17).
Liver Cancer
LoC platforms are also being adapted to model hepatocellular carcinoma (HCC). By co-culturing tumor-derived hepatocytes or patient-derived cancer cells with endothelial and immune cells, researchers recreate aspects of the tumor microenvironment, including vascular interactions and immune infiltration. These models are being used to study tumor progression and test immunotherapies such as checkpoint inhibitors in a human-relevant setting. Importantly, this approach links personalized medicine strategies with organ-on-chip technologies, as patient-derived tumor samples can inform individualized therapeutic testing(18).
In a nutshell
Liver-on-chip systems have moved decisively beyond proof-of-concept. Their capacity to sustain metabolic activity, replicate immune and fibrotic responses, and integrate with other organ chips makes them highly versatile platforms. In drug metabolism and pharmacokinetics (DMPK), they provide more accurate IVIVE data. In safety assessment, they offer the most advanced preclinical tool for predicting DILI and dissecting mechanisms of hepatotoxicity. In drug–drug interactions, they allow systemic modeling of metabolite-mediated effects. And in disease modeling, they enable unprecedented investigations into NAFLD/NASH, viral hepatitis, and liver cancer.
By delivering data that are more human-relevant and mechanistically informative, LoC platforms align with the industry’s drive toward New Approach Methodologies (NAMs) and alternatives to animal testing. As pharmaceutical companies seek to reduce attrition and accelerate pipelines, liver-on-chip is emerging as a critical component of a modern, human-centered approach to drug discovery and therapeutic development.
From Validation to Adoption in Pharma
The field of liver-on-chip (LoC) technology is advancing rapidly, fueled by breakthroughs in 3D cell culture, microfabrication, and computational biology. What began as a proof-of-concept has evolved into a powerful translational platform now entering the mainstream of pharmaceutical drug discovery. This chapter highlights the most recent advances, key challenges, and the outlook for widespread adoption, spanning technical developments, regulatory acceptance, and future integration with personalized medicine and regenerative therapies.
State of the Art: Expanding the Frontier
Materials and fabrication innovations
Polydimethylsiloxane (PDMS) has been the workhorse material for prototyping organ-on-chip devices, but it presents issues such as drug absorption that compromise pharmacokinetic accuracy. Recent years have seen a shift toward polymers such as cyclic olefin copolymer (COC) and polymethyl methacrylate (PMMA), which are more compatible with high-throughput manufacturing (e.g., injection molding) and reduce compound loss. In parallel, 3D printing and digital light processing (DLP) allow rapid prototyping of more complex architectures, enabling customizable designs that reflect disease-specific biology(19).
Complex multi-organ systems
The frontier of organ-on-chip research now lies in integrated multi-organ configurations. Beyond gut–liver or liver–kidney systems, platforms connecting three or more organ models are being reported. These networks enable holistic investigation of systemic drug distribution, metabolism, and toxicity. For example, linking liver, kidney, and heart chips allows researchers to assess how hepatic metabolites alter renal clearance or cardiotoxic risk, a step toward capturing whole-body pharmacology in vitro.
Integration with AI/ML
Liver chips generate vast amounts of complex data, from biosensor readouts to high-resolution imaging and multi-omics profiles. The application of artificial intelligence (AI) and machine learning (ML) is emerging as a game-changer for extracting insights. Algorithms can identify early biomarkers of hepatotoxicity, optimize chip protocols, and support in vitro–in vivo extrapolation (IVIVE) by integrating chip data into physiologically based pharmacokinetic (PBPK) models.
New frontiers in nanomedicine
The unique physicochemical properties of nanoparticles challenge traditional preclinical models. Liver-on-chip is increasingly used to assess nanomedicine biodistribution, clearance, and toxicity under physiologically relevant flow conditions, offering a predictive tool for this growing therapeutic class.
Overcoming Key Hurdles: The Path to Industrialization
Despite impressive progress, several challenges must be addressed for LoC to mature into a standard industrial tool:
- Vascularization. Replicating the fenestrated sinusoidal network of the liver remains a bioengineering challenge. Stable vascular interfaces are needed to sustain nutrient and oxygen delivery and to accurately model vascular diseases or fibrosis.
- Long-term functional stability. Although LoC systems extend hepatocyte viability beyond 28 days, gradual dedifferentiation limits their application to chronic disease and low-dose toxicity studies.
- Standardization and reproducibility. Variability in chip design, fabrication, culture media, and iPSC-derived hepatocyte quality remains a barrier. Industry-wide consensus guidelines, such as those proposed by the IQ MPS consortium, will be essential for regulatory trust.
- Material issues. Absorption of hydrophobic drugs into PDMS and other polymers can distort exposure profiles. Surface treatments, alternative polymers, and robust controls are being developed to mitigate this risk.
Addressing these bottlenecks will determine how quickly LoC transitions from a “technology-push” innovation to a “demand-pull” solution aligned with pharmaceutical and regulatory needs.
Toward Regulatory Acceptance
Regulatory recognition is pivotal for mainstream adoption. The passage of the FDA Modernization Act 2.0 and 3.0 in 2022 and 2025 respectively, explicitly encourages consideration of New Approach Methodologies (NAMs), including organ-on-chip, as alternatives to animal testing. Programs such as FDA ISTAND (Innovative Science and Technology Approaches for New Drugs) and collaborative consortia like IQ MPS are actively qualifying chip platforms for specific contexts of use.
The current regulatory strategy emphasizes targeted validation. Rather than replacing animal testing wholesale, the focus is on areas where LoC offers clear advantages, for instance, DILI prediction. Success in these narrow but critical use cases builds confidence for gradual expansion to broader applications in drug safety and efficacy testing.
Future Outlook: Personalized Medicine and Regenerative Therapies
The convergence of liver-on-chip, iPSC-derived hepatocytes, and AI/ML opens transformative possibilities:
Patient-on-a-Chip. By deriving hepatocytes and supporting cell types from an individual’s induced pluripotent stem cells (iPSCs), researchers can build personalized liver models that capture genetic polymorphisms or disease backgrounds. This enables testing of multiple drugs on a “miniature patient” before clinical administration, reducing adverse events and guiding therapy selection—an essential step toward personalized medicine.
Digital twins. Personalized chip data can feed into computational models to create “digital twins”: virtual patients capable of simulating drug response and disease progression. These models may revolutionize trial design, risk stratification, and chronic disease management.
Regenerative medicine. Advances in chip-based tissue engineering may eventually support the creation of vascularized liver grafts for transplantation. By scaling the principles used in LoC, it may become possible to fabricate implantable tissues using a patient’s own cells, offering a solution to organ shortages while mitigating rejection risks.
Conclusion
Liver-on-chip technology exemplifies the promise of bioconvergence, the integration of biology, engineering, and data science to create predictive and clinically relevant models. By combining 3D cell culture, multicellular co-culture, microfluidics, and real-time sensing, LoC platforms surpass the limitations of traditional assays and offer a human-relevant alternative to animal testing.
Many applications showcase the potential of LoC; validated DILI prediction, improved DMPK profiling, mechanistic insights into drug–drug interactions, and novel models for NAFLD, NASH, HBV, and liver cancer. Looking ahead, overcoming technical challenges, establishing standards, and securing regulatory trust will be decisive for broad industrial adoption.
As the field matures, liver-on-chip is positioned not only as a translational research tool but as a cornerstone of future drug discovery, personalized medicine, and even regenerative therapy. For the pharmaceutical industry, it offers a tangible opportunity to reduce attrition, improve patient safety, and accelerate the delivery of next-generation therapeutics.
References
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting Organ-Level Lung Functions on a Chip. Science. 25 juin 2010;328(5986):1662‑8.
- Deng J, Wei W, Chen Z, Lin B, Zhao W, Luo Y, et al. Engineered Liver-On-A-Chip Platform to Mimic Liver Functions and Its Biomedical Applications: A Review. Micromachines. 7 oct 2019;10(10):676.
- Ma LD, Wang YT, Wang JR, Wu JL, Meng XS, Hu P, et al. Design and fabrication of a liver-on-a-chip platform for convenient, highly efficient, and safe in situ perfusion culture of 3D hepatic spheroids. Lab Chip. 2018;18(17):2547‑62.
- Weng Y, Chang S, Shih M, Tseng S, Lai C. Scaffold‐Free Liver‐On‐A‐Chip with Multiscale Organotypic Cultures. Adv Mater. sept 2017;29(36):1701545.
- Jang KJ, Otieno MA, Ronxhi J, Lim HK, Ewart L, Kodella KR, et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med. 6 nov 2019;11(517):eaax5516.
- Tilles AW, Baskaran H, Roy P, Yarmush ML, Toner M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat‐plate bioreactor. Biotechnol Bioeng. 5 juin 2001;73(5):379‑89.
- Yang Z, Liu X, Cribbin EM, Kim AM, Li JJ, Yong KT. Liver-on-a-chip: Considerations, advances, and beyond. Biomicrofluidics. 1 déc 2022;16(6):061502.
- Docci L, Milani N, Ramp T, Romeo AA, Godoy P, Franyuti DO, et al. Exploration and application of a liver-on-a-chip device in combination with modelling and simulation for quantitative drug metabolism studies. Lab Chip. 2022;22(6):1187‑205.
- Frojdenfal S, Zuchowska A. Advanced Liver-on-a-Chip Model for Evaluating Drug Metabolism and Hepatotoxicity. Biosensors. 6 sept 2024;14(9):435.
- Wang M, Sasaki Y, Sakagami R, Minamikawa T, Tsuda M, Ueno R, et al. Perfluoropolyether-Based Gut-Liver-on-a-Chip for the Evaluation of First-Pass Metabolism and Oral Bioavailability of Drugs. ACS Biomater Sci Eng. 8 juill 2024;10(7):4635‑44.
- Ewart L, Apostolou A, Briggs SA, Carman CV, Chaff JT, Heng AR, et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun Med. 6 déc 2022;2(1):154.
- Kulsharova G, Kurmangaliyeva A. Liver microphysiological platforms for drug metabolism applications. Cell Prolif. sept 2021;54(9):e13099.
- Slaughter VL, Rumsey JW, Boone R, Malik D, Cai Y, Sriram NN, et al. Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical therapeutic efficacy evaluation. Sci Rep. 23 juin 2021;11(1):13159.
- Kang Y, Rawat S, Duchemin N, Bouchard M, Noh M. Human Liver Sinusoid on a Chip for Hepatitis B Virus Replication Study. Micromachines. 20 janv 2017;8(1):27.
- Ortega-Prieto AM, Skelton JK, Wai SN, Large E, Lussignol M, Vizcay-Barrena G, et al. 3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus infection. Nat Commun. 14 févr 2018;9(1):682.
- Carvalho V, Ferreira M, Rodrigues RO, Teixeira SFCF, Lima RA. Computational and experimental advances in liver-on-a-chip technology for cancer research: a systematic review. Biophys Rev. févr 2025;17(1):151‑67.
FAQ
The concept of organ-on-a-chip (OoC) technology was formed in the early 2000s. It came from the convergence of microfabrication, microfluidics, and cell culture. The main objective was to move past static three-dimensional cultures. Researchers wanted to design microenvironments that were continuously perfused. These new systems were intended to be capable of replicating organ-level functions in vitro. A landmark publication in 2010 demonstrated this approach with a lung-on-a-chip. This device recreated the alveolar–capillary interface. It also incorporated cyclic stretching to mimic breathing. This achievement showed that OoC systems could capture dynamic mechanical and biochemical functions.
Early liver-on-chip models were designed to address a known problem. Rapid loss of function is observed in two-dimensional hepatocyte cultures. Microfabrication strategies were used. One such strategy was micropatterning hepatocytes with endothelial or stromal cells. This method was shown to increase intercellular communication. It also substantially increased cytochrome P450 (CYP450) activity when compared to random co-cultures. Microfluidic platforms were introduced later. These designs allowed the co-culture of hepatocytes and liver sinusoidal endothelial cells. This configuration reflected the hepatic sinusoid. Other designs used hexagonal chamber geometries. These were meant to approximate the lobular organisation of the liver. These efforts extended hepatocyte viability.
Primary human hepatocytes (PHHs) are considered the benchmark for physiological relevance. In conventional culture, PHHs lose function quickly. Their performance is different within liver-on-chip systems. In these devices, they receive perfusion and exist in a three-dimensional context. They are also co-cultured with non-parenchymal cells. These conditions allow them to sustain albumin and urea secretion. Transporter activity and stable CYP450 expression are also maintained for weeks. It was demonstrated that species-specific liver chips containing PHHs could replicate known interspecies differences in drug-induced liver injury. This finding highlights their translational value for studies.
The defining feature of organ-on-a-chip systems is the use of microfluidics. This is the technology of manipulating fluids in very small channels. In liver-on-chip (LoC) platforms, these channels act as an artificial vascular network. They permit the continuous perfusion of culture medium. This perfusion simulates the constant flow of blood through the liver sinusoids. This dynamic flow ensures a steady supply of oxygen and nutrients. It also removes metabolic waste products. This process is important for maintaining the health of high-density liver cell cultures over long periods. Perfusion also allows for precise control over the cellular microenvironment.
A main consequence of continuous, unidirectional flow is the natural establishment of chemical gradients. These gradients run along the length of the microchannel. As medium flows past the cells, oxygen and nutrients are consumed. Metabolites are released at the same time. This creates a gradient from the inlet to the outlet. This process mimics the zonation that occurs in the native liver lobule. In the lobule, hepatocytes in “zone 1” (periportal) are exposed to highly oxygenated, nutrient-rich blood. They have different metabolic functions than hepatocytes in “zone 3” (pericentral), which see lower oxygen levels. The ability of LoC to induce this zonation in vitro is a major step.
The transition from flat 2D surfaces to a 3D culture environment is a requirement for physiological relevance. In the native liver, hepatocytes are organised into complex structures. They are in constant communication with nearby cells and the extracellular matrix (ECM). This 3D context is needed for maintaining hepatocyte polarity. Polarity is the state of having distinct apical and basolateral surfaces. This is required for bile secretion and nutrient uptake. The 3D context also supports cuboidal morphology and high-level metabolic function. Many LoC designs use biocompatible hydrogels, like collagen or Matrigel, as scaffolds. These provide structural support.
The liver is a complex, multicellular organ. Hepatocytes (parenchymal cells) work closely with non-parenchymal cells (NPCs). Important NPCs include liver sinusoidal endothelial cells (LSECs), which line the sinusoids. Kupffer cells, the resident macrophages, are also involved in immunity. Hepatic stellate cells (HSCs) are the primary source of scar tissue during fibrosis. To improve physiological relevance, an LoC must be a multicellular system. Co-culturing hepatocytes with NPCs is needed to stabilise hepatocyte function. This occurs through paracrine signaling and direct cell-to-cell contacts. The inclusion of specific NPCs is tailored to the research question.
A limitation of traditional cell culture is its reliance on end-point assays. These assays are often destructive. They provide only a single snapshot in time. An emerging feature of LoC technology is the ability to integrate miniaturised biosensors directly into the chip. This permits continuous, real-time, and non-invasive monitoring of the tissue’s health and metabolic activity. For example, electrodes can be integrated to measure transepithelial/transendothelial electrical resistance (TEER). TEER provides a quantitative measure of cellular barrier integrity. Other sensors can monitor the oxygen consumption rate (OCR). This capability allows researchers to observe the full dynamic response to a drug exposure.
One mature application for LoC is in safety pharmacology. This is particularly true for drug-induced liver injury (DILI). Traditional preclinical models, including animal testing, have failed to predict hepatotoxicity in humans. A validation study tested 870 Liver-Chips with 27 compounds of known clinical outcomes. The platform correctly identified hepatotoxic drugs with 87% sensitivity and 100% specificity. This performance outperformed animal models. The compounds tested were previously classified as safe in animals but later caused severe liver injury in patients. LoC platforms also allow for the mechanistic dissection of liver injury. Specific assays can distinguish between apoptosis, steatosis, or cholestasis.
LoC platforms are used to study the pathophysiology of human liver diseases. They have been effective at replicating the pathological features of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH). The initial stage, steatosis, is induced by perfusing liver cells with medium containing high concentrations of free fatty acids. To model the progression to NASH, which involves inflammation and fibrosis, researchers introduce pro-inflammatory stimuli. The inclusion of Kupffer cells and hepatic stellate cells is needed for this. These “NASH-on-a-chip” models exhibit disease phenotypes. These include hepatocyte injury, lipid accumulation, inflammation, and collagen deposition. They provide a platform for testing anti-fibrotic drug candidates.