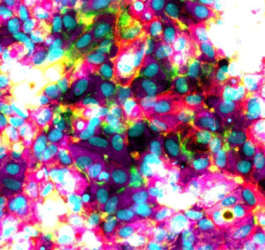
The liver organoid is transforming how researchers approach drug discovery and safety testing. As miniature, three-dimensional models grown from human cells, liver organoids replicate key metabolic and detoxification functions of the liver far more accurately than traditional 2D cultures or animal models. These advanced 3D cell culture systems sustain hepatocyte activity, enable chronic toxicity testing, and provide a powerful platform to study drug metabolism, drug–drug interactions, and patient-specific responses. By offering a human-relevant alternative to animal testing, liver organoids are emerging as essential tools for developing safer, more effective therapies and advancing personalized medicine.
Learn more about our ready to use human liver organoid models.
The Liver’s Central Role in Drug Discovery
The liver is the body’s central hub for processing xenobiotics and remains one of the most important organs to model in drug discovery. Almost every therapeutic compound (whether a small molecule or biologic) interacts with the liver in some way. Hepatocytes, the predominant liver cells, are loaded with cytochrome P450 (CYP450) enzymes and transporters that together drive drug metabolism and detoxification. This metabolic machinery governs the absorption, distribution, metabolism, and excretion (ADME) profile of a drug, influencing its bioavailability, therapeutic window, and long-term safety.
The liver’s dual nature is both an asset and a liability in pharmacology. On one hand, it can activate prodrugs into their therapeutic forms. For example, tegafur is metabolized into 5-fluorouracil, an effective anticancer agent. On the other hand, the same enzymatic processes can generate reactive metabolites that damage hepatocytes, triggering drug-induced liver injury (DILI). DILI is one of the leading causes of clinical trial attrition and post-marketing drug withdrawals (estimated to account for 13% of failure in trials(1)), underscoring how critical it is to predict liver responses early in development.
Beyond metabolism and detoxification, the liver is also a key site for drug–drug interactions (DDIs). Because many drugs are metabolized by the same CYP enzyme families, concurrent medications may compete or interfere, altering drug plasma concentrations and leading to unexpected toxicity or loss of efficacy. This is particularly important in therapeutic areas where polypharmacy is common, such as oncology, infectious disease, and cardiology. The liver’s role in first-pass metabolism further complicates matters: orally administered drugs are processed in the liver before reaching systemic circulation, which can drastically reduce bioavailability or generate metabolites with distinct pharmacological effects.
For decades, the pharmaceutical industry has relied on a combination of 2D cell culture assays and animal models to study these processes. While indispensable in their time, both approaches have significant limitations:
- Two-dimensional hepatocyte cultures: Primary human hepatocytes lose their polarity and metabolic activity within days when grown in flat monolayers. This rapid dedifferentiation makes them poorly suited for long-term studies, chronic toxicity testing, or evaluation of delayed adverse events.
- Immortalized cell lines: Cell lines like HepG2 are more robust but often lack physiologically relevant expression of CYP enzymes and transporters. Their utility in predicting human metabolism is therefore limited.
- Animal models: Rodent and non-human primate studies provide whole-organism context but often fail to capture human-specific metabolism and toxicity. Species differences in enzyme expression and metabolic pathways mean drugs that appear safe in animals may cause liver injury in humans, or conversely, safe drugs may be abandoned prematurely due to misleading animal toxicity.
The consequence of these limitations is a predictive gap: too many drug candidates clear preclinical testing only to fail in clinical trials due to hepatotoxicity, poor pharmacokinetics, or unforeseen DDIs. This gap is not only a scientific challenge but also a financial one. Each late-stage failure costs hundreds of millions of dollars and can erode confidence in therapeutic pipelines.
Recognizing these shortcomings, regulatory agencies and industry stakeholders have called for New Approach Methodologies (NAMs) that provide more human-relevant insights and serve as alternatives to animal testing. Among the most promising are 3D cell culture systems, particularly liver organoids and spheroids. By recreating the architecture and multicellular complexity of the liver in vitro, these models sustain hepatocyte functionality for extended periods and offer a more predictive window into human drug responses. In the next chapter, we will explore how liver organoids and spheroids are built, what they can measure, and why they are becoming essential tools in modern drug discovery.
3D Liver Organoids and Spheroids Explained
The shortcomings of traditional assays and animal models have accelerated the adoption of 3D cell culture approaches designed to capture the complexity of the human liver. Among these, liver organoids and spheroids have emerged as some of the most impactful innovations in preclinical research. These miniature liver models combine cellular diversity, physiological function, and experimental control, making them increasingly central to modern drug discovery.
What are liver organoids and spheroids?
At their core, both organoids and spheroids are three-dimensional liver tissues grown in vitro. They differ mainly in origin:
- Spheroids are typically formed by clustering primary human hepatocytes into compact, spherical aggregates. Sometimes these are combined with supportive stromal or endothelial cells to sustain function.
- Organoids are more complex, often derived from pluripotent stem cells (induced pluripotent or embryonic) or adult liver progenitors. Under the right conditions, these cells self-organize into tissue-like structures that mimic aspects of liver architecture, sometimes including bile duct–like structures or non-parenchymal cells.
In both cases, the 3D architecture is critical. Unlike 2D cultures, where hepatocytes rapidly lose polarity and dedifferentiate, organoids and spheroids maintain cell–cell and cell–matrix interactions. This microenvironment supports hepatocyte viability and function for weeks, if not months, making them far more suitable for long-term studies.
Why do 3D models matter for drug discovery?
In the context of preclinical development, organoids and spheroids offer several advantages:
- Sustained metabolism: They retain cytochrome P450 activity and conjugation pathways, enabling accurate Phase I and II metabolism studies.
- Functional biomarkers: Stable secretion of albumin and urea provides readouts of liver health and function over extended cultures.
- Transporter activity: Expression of clinically relevant transporters (e.g., BSEP, OATP) makes these systems suitable for evaluating drug disposition and cholestatic liabilities.
- Multicellular responses: Incorporating Kupffer macrophages and stellate cells allows organoids to capture immune activation, cytokine release, and fibrotic remodeling—key components of drug-induced liver injury (DILI).
Because these functions are preserved, 3D liver models enable studies that are difficult or impossible in flat monolayers. Repeated-dose toxicity testing can reveal cumulative or delayed effects. Drug–drug interaction (DDI) studies become more predictive because enzyme induction or inhibition can be tracked over time. Even subtle metabolic shifts such as changes in mitochondrial function or glutathione depletion can be detected in physiologically relevant settings.
Applications across pharma and personalized medicine
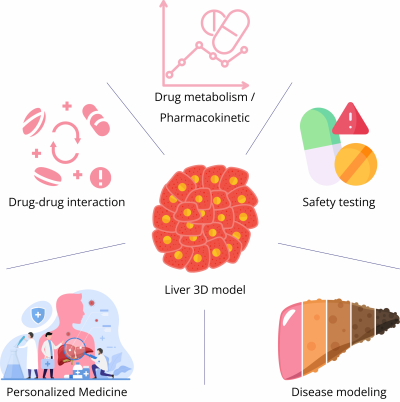
The utility of liver organoids spans several domains:
- Drug metabolism and pharmacokinetics (DMPK): 3D cultures are used to calculate intrinsic clearance rates, characterize metabolite formation, and evaluate prodrug activation.
- DDI assessment: By profiling CYP induction/inhibition and transporter inhibition, organoids help predict whether new drugs will interfere with existing therapies.
- Safety testing: Long-term viability makes organoids ideal for detecting idiosyncratic hepatotoxicity or fibrosis that emerges only after repeated exposure.
- Disease modeling: Researchers have engineered organoids to mimic conditions such as non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), enabling efficacy testing of metabolic drugs in a human-relevant system.
Personalized medicine: Perhaps most excitingly, organoids can be generated from patient-derived induced pluripotent stem cells. These retain the donor’s genetic background, offering a platform to test therapies in a patient-specific manner. For rare genetic liver diseases or variable drug responses across populations, this could transform clinical trial design and therapeutic selection.
Strengths and current limitations
For pharmaceutical scientists, organoids represent a more predictive and human-relevant alternative to animal testing. They are miniaturized, scalable, and increasingly compatible with automation and medium-throughput workflows. Their ability to sustain function over weeks provides a window into chronic responses that were previously inaccessible.
However, challenges remain. Variability between batches and across laboratories can affect reproducibility, as organoid formation is sensitive to culture conditions and growth factor cocktails. Standardization and quality control are active areas of development, particularly as companies seek regulatory acceptance. Scaling up production for high-throughput screening is another hurdle, though advances in bioreactors and automated platforms are addressing this need.
A bridge to better translation
In short, 3D liver organoids and spheroids provide a critical bridge between oversimplified 2D cultures and whole-animal studies. They capture the structural and functional hallmarks of the human liver, support a wide range of pharmacological assays, and open doors to personalized medicine approaches. While they are not a replacement for every model, they are a strategic complement that helps de-risk drug candidates earlier in development, ultimately reducing attrition and improving translation from bench to clinic.
Applications and Case Studies in Drug Discovery
The promise of liver organoids and spheroids is best illustrated by their real-world applications. Over the past decade, these 3D cell culture systems have moved from proof-of-concept experiments in academic labs to tools actively considered by pharmaceutical companies to de-risk development programs. By providing more physiologically relevant insights, they are helping drug developers bridge the critical gap between preclinical studies and human trials.
Predicting hidden drug-induced liver injury
Perhaps the most striking evidence of impact comes from studies on drug-induced liver injury (DILI), a leading cause of drug failure. In 2023, researchers reported that liver organoids derived from human pluripotent stem cells successfully flagged a dangerous drug–drug interaction between tenofovir alafenamide (TAF) and inarigivir. Conventional hepatocyte assays and animal models had not identified any issues, but the organoids revealed significant hepatotoxicity when the compounds were combined. Unfortunately, this toxicity later manifested in clinical trials, resulting in severe patient outcomes. Had organoid testing been integrated earlier, the risk might have been recognized before patient exposure. This case exemplifies why organoids are seen as an essential complement to existing preclinical models in drug discovery(2).
Chronic toxicity and fibrosis studies
Another advantage of organoids is their long-term viability, which enables repeated-dose and chronic exposure studies. Traditional 2D hepatocyte cultures deteriorate after just a few days, limiting their use in studying delayed toxicities. In contrast, organoids and spheroids can be cultured for weeks while maintaining enzyme activity and secretory function(3). This stability allows researchers to detect cumulative hepatotoxicity, fibrotic changes, and subtle metabolic perturbations that mimic clinical scenarios. When stellate cells are incorporated, organoids can even model the onset of fibrosis, providing a human-relevant platform to evaluate anti-fibrotic therapies(4,5).
Modeling complex diseases in vitro
Beyond toxicity, liver organoids have been engineered to replicate hallmarks of chronic liver disease. Models of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) have been created by inducing lipid accumulation and inflammatory signaling within 3D cultures. These organoids reproduce the interplay of hepatocytes, Kupffer cells, and stellate cells that drives steatosis and fibrotic progression in patients(6). For pharma companies developing drugs against NAFLD or NASH (a therapeutic area with a high failure rate) such organoid models offer a valuable way to test efficacy and mechanism in a controlled, human-specific system before advancing to the clinic.
Enabling personalized medicine
One of the most exciting frontiers is the use of patient-derived liver organoids. By generating organoids from induced pluripotent stem cells reprogrammed from patient samples, scientists can create models that retain the donor’s genetic background(7). This enables the study of how individual variability in CYP enzymes, transporters, or disease mutations influences drug metabolism and toxicity. For rare liver disorders, oncology, or subpopulations with unique genetic polymorphisms, such organoids provide a path to personalized medicine. They can help identify patients most likely to benefit from a therapy (or those at higher risk of adverse effects) before treatment begins. In the long run, this approach could transform how clinical trials are designed and how precision therapies are delivered.
Toward regulatory acceptance and reduced animal use
As evidence accumulates, regulatory agencies are beginning to acknowledge the potential of organoids as part of the New Approach Methodologies (NAMs) portfolio. Their use aligns with the global push to find alternatives to animal testing, a shift driven by ethical considerations, regulatory changes, and the recognition that animal data often fails to predict human outcomes. While organoids are not yet a universal standard, case studies continue to demonstrate their ability to provide more predictive, human-relevant data that complements or replaces certain animal studies(8,9).
A strategic fit in the drug discovery workflow
For pharmaceutical companies, the question is not whether organoids will replace existing models, but where they add the most value. Current best practice is to integrate organoids into a tiered testing strategy: high-throughput assays and computational screens are used to triage large libraries, organoids are applied to investigate DMPK and toxicity questions in detail, and organ-on-chip or multi-organ platforms are employed when systemic interactions are critical. In this workflow, organoids function as an early warning system, identifying liabilities that might otherwise only appear in costly late-stage studies or, worse, in patients.
Looking ahead
As liver organoid research continues to mature, the next frontier is not only scientific innovation but also standardization and scalability. For pharmaceutical adoption, reproducibility across laboratories, automated production, and harmonized quality control will be critical. The ability to generate organoids at consistent quality and at industrial scale will determine whether they can become routine tools in drug discovery pipelines rather than niche research assets. Regulatory acceptance is also on the horizon, as agencies increasingly recognize New Approach Methodologies (NAMs) and seek alternatives to animal testing that provide more predictive, human-relevant insights.
At the same time, organoid models themselves are becoming more physiologically relevant thanks to advances in stem cell biology. A recent study by Igarashi et al. (2025) demonstrated that human adult hepatocyte organoids can be generated and expanded long-term while maintaining a stable hepatic identity. By carefully modulating Wnt and STAT3 signaling, the authors overcame the problem of ductal metaplasia and produced organoids with functional bile canaliculi networks, robust CYP450 activity, and intact urea and lipid metabolism. These organoids capture the hallmarks of mature adult liver tissue, narrowing the gap between in vitro systems and human physiology(10).
Complementing this, Reza et al. (2025) reported the generation of multi-zonal liver organoids from pluripotent stem cells. By priming progenitors with factors such as ascorbate and bilirubin, they achieved self-organization into periportal, interzonal, and pericentral compartments, recapitulating the liver’s intrinsic metabolic gradients. These organoids exhibited zone-specific CYP450 activity, urea cycle function, and differential sensitivity to hepatotoxins such as acetaminophen and allyl alcohol. This represents a breakthrough in modeling liver zonation, a critical determinant of drug metabolism and drug-induced liver injury(11).
Together, these advances suggest that liver organoids are evolving from simplified proxies into highly nuanced human liver models capable of capturing maturity, zonation, and have the potential to study multi-organ crosstalk when used in microfluidic chip device (liver-on-chip). When combined with ongoing progress in automation and regulatory frameworks, they position organoids as indispensable tools for the future of drug discovery, personalized medicine, and safer therapeutic development.
References
- Redfern W, Ewart L, Hammond T, Bialecki R, Kinter L, Lindgren S. Impact and frequency of different toxicities throughout the pharmaceutical life cycle. The Toxicologist. 1 janv 2010;114.
- Zhang CJ, Meyer SR, O’Meara MJ, Huang S, Capeling MM, Ferrer-Torres D, et al. A human liver organoid screening platform for DILI risk prediction. J Hepatol. mai 2023;78(5):998‑1006.
- Hendriks DFG, Fredriksson Puigvert L, Messner S, Mortiz W, Ingelman-Sundberg M. Hepatic 3D spheroid models for the detection and study of compounds with cholestatic liability. Sci Rep. 19 oct 2016;6(1):35434.
- Lee H, Mun SJ, Jung C, Kang H, Kwon J, Ryu J, et al. In vitro modeling of liver fibrosis with 3D co‐culture system using a novel human hepatic stellate cell line. Biotechnol Bioeng. mai 2023;120(5):1241‑53.
- Wu X, Jiang D, Yang Y, Li S, Ding Q. Modeling drug-induced liver injury and screening for anti-hepatofibrotic compounds using human PSC-derived organoids. Cell Regen. 3 mars 2023;12(1):6.
- Ströbel S, Kostadinova R, Fiaschetti-Egli K, Rupp J, Bieri M, Pawlowska A, et al. A 3D primary human cell-based in vitro model of non-alcoholic steatohepatitis for efficacy testing of clinical drug candidates. Sci Rep. 23 nov 2021;11(1):22765.
- Ariño S, Ferrer-Lorente R, Serrano G, Zanatto L, Martínez-García De La Torre RA, Gratacós-Ginès J, et al. Patient-derived liver organoids recapitulate liver epithelial heterogeneity and enable precision modeling of alcohol-related liver disease. J Hepatol. août 2025;S0168827825023803.
- Han DW, Xu K, Jin ZL, Xu YN, Li YH, Wang L, et al. Customized liver organoids as an advanced in vitro modeling and drug discovery platform for non-alcoholic fatty liver diseases. Int J Biol Sci. 2023;19(11):3595‑613.
- Shao W, Xu H, Zeng K, Ye M, Pei R, Wang K. Advances in liver organoids: replicating hepatic complexity for toxicity assessment and disease modeling. Stem Cell Res Ther. 26 janv 2025;16(1):27.
- Igarashi R, Oda M, Okada R, Yano T, Takahashi S, Pastuhov S, et al. Generation of human adult hepatocyte organoids with metabolic functions. Nature [Internet]. 16 avr 2025 [cité 13 mai 2025]; Disponible sur: https://www.nature.com/articles/s41586-025-08861-y
- Reza HA, Santangelo C, Iwasawa K, Reza AA, Sekiya S, Glaser K, et al. Multi-zonal liver organoids from human pluripotent stem cells. Nature [Internet]. 16 avr 2025 [cité 13 mai 2025]; Disponible sur: https://www.nature.com/articles/s41586-025-08850-1
FAQ
The liver is the body’s central hub for processing xenobiotics. Almost every therapeutic compound interacts with the liver. Hepatocytes, the main liver cells, are equipped with cytochrome P450 (CYP450) enzymes and transporters. Drug metabolism and detoxification are driven by this machinery. A drug’s absorption, distribution, metabolism, and excretion (ADME) profile is governed by these processes. Bioavailability and safety are influenced by this profile. The liver’s processing can be beneficial. Prodrugs, for example, can be activated into their therapeutic forms. Tegafur is metabolised into 5-fluorouracil, an anticancer agent. This dual nature makes the liver a primary organ to model in drug development.
Drug-induced liver injury, or DILI, is a negative outcome of the liver’s metabolic processes. While the liver’s enzymes can activate drugs, reactive metabolites can also be generated. Damage to hepatocytes is caused by these substances. DILI is noted as one of the leading reasons for clinical trial attrition. It is also a cause of post-marketing drug withdrawals. An estimated 13% of trial failures are accounted for by DILI. Because of this, the prediction of liver responses early in development is considered necessary. Reliable models are needed to foresee these toxic reactions before a drug reaches patients.
Two-dimensional hepatocyte cultures have known deficiencies. When primary human hepatocytes are grown in flat monolayers, polarity is lost. Metabolic activity is also lost within days. This rapid change is called dedifferentiation. Because of this, the cultures are considered poorly suited for long-term studies. The evaluation of delayed adverse events or chronic toxicity testing cannot be reliably performed. Immortalized cell lines, such as HepG2, are more stable. Physiologically relevant expression of CYP enzymes and transporters is often absent in these lines. Their usefulness in predicting human metabolism is therefore restricted. These deficiencies have led to a predictive gap in preclinical testing.
A whole-organism context is provided by animal models. Rodent and non-human primate studies are common. Human-specific metabolism and toxicity are often not captured by these models. This failure is caused by species differences in enzyme expression and metabolic pathways. A drug that appears safe in animals might cause liver injury in humans. The reverse can also be true. Safe drugs may be abandoned prematurely because of misleading animal toxicity data. A predictive gap is created by these limitations. Too many drug candidates that clear preclinical testing later fail in clinical trials due to hepatotoxicity. This situation has prompted calls for alternative testing methods that are more human-relevant.
Organoids and spheroids are both three-dimensional liver tissues grown in vitro. Their main difference is their origin. Spheroids are typically formed when primary human hepatocytes are clustered together. These are formed into compact, spherical aggregates. Supportive stromal or endothelial cells are sometimes combined with them to sustain function. Organoids are described as being more intricate. They are often derived from pluripotent stem cells, which can be induced or embryonic. Adult liver progenitors can also be used. When given the right conditions, self-organisation of these cells occurs. Tissue-like structures are formed that mimic aspects of liver architecture, sometimes including bile duct–like formations.
The three-dimensional architecture is a defining feature of these models. In 2D cultures, polarity is rapidly lost by hepatocytes and dedifferentiation occurs. In contrast, cell-cell and cell-matrix interactions are maintained in organoids and spheroids. This microenvironment is supportive. Hepatocyte viability and function are supported for weeks or even months. The 3D models are therefore far more suitable for long-term studies. Because functions are preserved, studies that are difficult in flat monolayers can be performed. For example, repeated-dose toxicity testing can be conducted. Cumulative or delayed effects can be shown by this testing. Sustained metabolism, including Phase I and II pathways, is retained. Functional biomarkers like albumin secretion are also stable.
The liver is a complex, multicellular organ. Hepatocytes (parenchymal cells) work closely with non-parenchymal cells (NPCs). Important NPCs include liver sinusoidal endothelial cells (LSECs), which line the sinusoids. Kupffer cells, the resident macrophages, are also involved in immunity. Hepatic stellate cells (HSCs) are the primary source of scar tissue during fibrosis. To improve physiological relevance, an LoC must be a multicellular system. Co-culturing hepatocytes with NPCs is needed to stabilise hepatocyte function. This occurs through paracrine signaling and direct cell-to-cell contacts. The inclusion of specific NPCs is tailored to the research question.
Beyond toxicity, liver organoids have been engineered to replicate hallmarks of chronic liver disease. Models of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) have been created. This is done by inducing lipid accumulation and inflammatory signaling within the 3D cultures. In these organoids, the interplay of different cell populations is reproduced. Hepatocytes, Kupffer cells, and stellate cells are included in this mix. Steatosis and fibrotic progression in patients are driven by this interplay. For companies developing drugs against NAFLD or NASH, a method for testing efficacy is offered by these organoid models. The mechanism of a drug can also be tested in a controlled, human-specific system before clinical advancement.
How do patient-derived organoids contribute to personalized medicine
One application for organoids is in personalized medicine. Organoids can be generated from induced pluripotent stem cells (iPSCs). These iPSCs are reprogrammed from patient samples. As a result, models are created that retain the donor’s genetic background. The study of how individual variability influences drug metabolism and toxicity is enabled by this. Variability in CYP enzymes, transporters, or disease mutations can be examined. For rare liver disorders or oncology, this is a path to individualized medicine. Patients who are most likely to benefit from a therapy can be identified. Patients at higher risk of adverse effects can also be identified before treatment begins. Clinical trial design could be changed by this approach.