Introduction
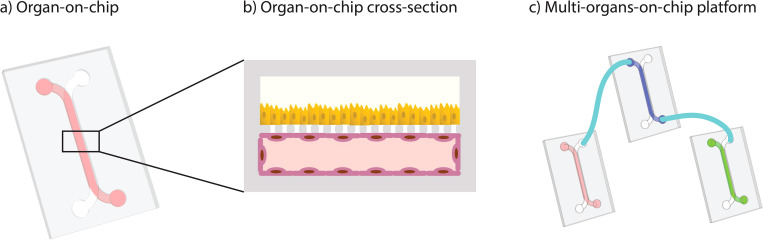
The authors present a simple and modular Microphysiological system without the use of complex components consisting of
1) a resistance module that controls the flow rate and
2) a physiologically relevant, three-dimensional blood vessel module. Flow is provided by an attached reservoir tank that feeds fluid into the resistance channel via hydrostatic pressure.
Optical Coherence Tomography (OCT) is used to measure fluid velocity at regions of interest.
Because of the system’s simplicity, it can simply be modified to include different microenvironmental components and to develop other organ-modeling systems. Due to the nature of the complexity of the modular microphysiological system, it is essential to have application-based systems so that, depending on the experiment, they can be versatile.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The author states that “Given the increased recognition of the importance of physiologically relevant microenvironments when designing in vitro assays, microphysiological systems (MPS) that mimic the critical function and structure of tissues and organs have gained considerable attention as alternatives to traditional experimental models.
Accordingly, the field is growing rapidly, and some promising MPS are being tested for use in pharmaceutical development and toxicological testing. However, most MPS are complex and require additional infrastructure, which limits their successful translation.
Here, we present a pumpless, modular MPS consisting of 1) a resistance module that controls the flow rate and 2) a physiologically relevant, three-dimensional blood vessel module. Flow is provided by an attached reservoir tank that feeds fluid into the resistance channel via hydrostatic pressure.
The flow rate is controlled by the height of the media in the tank and the resistance channel’s dimensions. The flow from the resistance module is streamed into the blood vessel module using a liquid bridge. We utilize optical coherence tomography (OCT) to measure fluid velocity at regions of interest.
The endothelial cells cultured in the MPS remain viable for up to 14 days and demonstrate the functional characteristics of the human blood vessels verified by tight junction expression and diffusion assay. Our results show that a modular MPS can simulate a functional endothelium in vitro while simplifying the operation of the MPS.
The simplicity of the system allows for modifications to incorporate other microenvironmental components and to build other organ-modeling systems easily.”
References
Tronolone JJ, Lam J, Agrawal A, Sung K. Pumpless, modular, microphysiological systems enabling tunable perfusion for long-term cultivation of endothelialized lumens. Biomed Microdevices. 2021 Apr 14;23(2):25. doi: 10.1007/s10544-021-00562-3. PMID: 33855605.
FAQ
A noted problem is the intricate nature of many microphysiological systems (MPS). Most systems have many parts. They also require additional equipment or infrastructure to operate. This intricacy limits their effective translation into wider use, such as in pharmaceutical development. This is a barrier to their adoption. The field has seen new systems being tested for use in toxicological testing. These systems are seen as alternatives to traditional models. This is because of the increased recognition of the value of physiologically relevant microenvironments. Still, the difficulty in operating them prevents their widespread application.
This system is modular and does not use a pump. It is made of two main parts. The first part is a resistance module. This module controls the rate of flow. The second part is a 3D blood vessel module that is physiologically relevant. Flow is supplied by a reservoir tank that is attached to the system. This tank feeds fluid into a resistance channel. The movement is caused by hydrostatic pressure. This means a pump is not required for operation. The flow from the resistance part is then streamed into the blood vessel part. This connection is made using a liquid bridge.
The rate of flow in this system is managed without any pumps. Instead, it is controlled by two main factors. The first factor is the height of the media within the attached reservoir tank. This height generates the hydrostatic pressure that moves the fluid. The second factor is the set of dimensions of the resistance channel. These dimensions provide a specific amount of resistance to the fluid. By adjusting these two factors, the rate of flow can be set. This design simplifies the operation of the microphysiological system. Fluid velocity can be measured at specific areas. This is done using optical coherence tomography, or OCT.
Yes, the system was tested with endothelial cells. These cells were cultured within the 3D blood vessel module. The cells were shown to remain viable for a period of up to 14 days. During this time, the cells displayed characteristics of working human blood vessels. This operational status was confirmed using two different methods. The first method was verifying the expression of tight junctions. The second method was performing a diffusion assay. The results indicate that this modular system can simulate a working endothelium in a laboratory setting. This is achieved while also simplifying the general operation of the MPS.