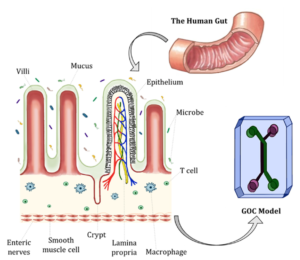
Understanding how the body processes fats is central to tackling obesity, type 2 diabetes, and fatty liver disease. Yet, traditional models often fail to capture the complexity of human lipid metabolism, which depends on the coordinated activity of organs like the liver, adipose tissue, pancreas, and muscle. Emerging technologies such as organoids for lipid metabolism and organ-on-chip platforms now make it possible to recreate these processes in vitro. In this article, we will first remind the role of metabolism-related organs and how they connect, then highlight new approaches and methodologies, from organoids to organ-on-chip, that are transforming disease modeling and drug discovery.
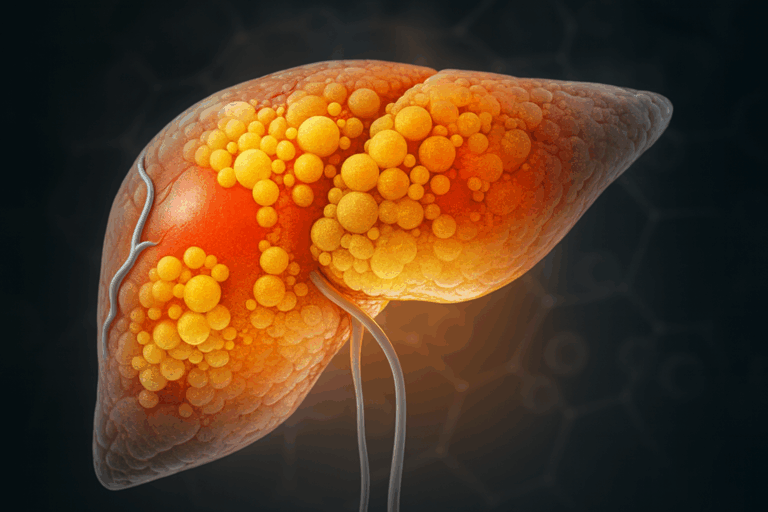
Learn more about our ready to use human liver organoid models.
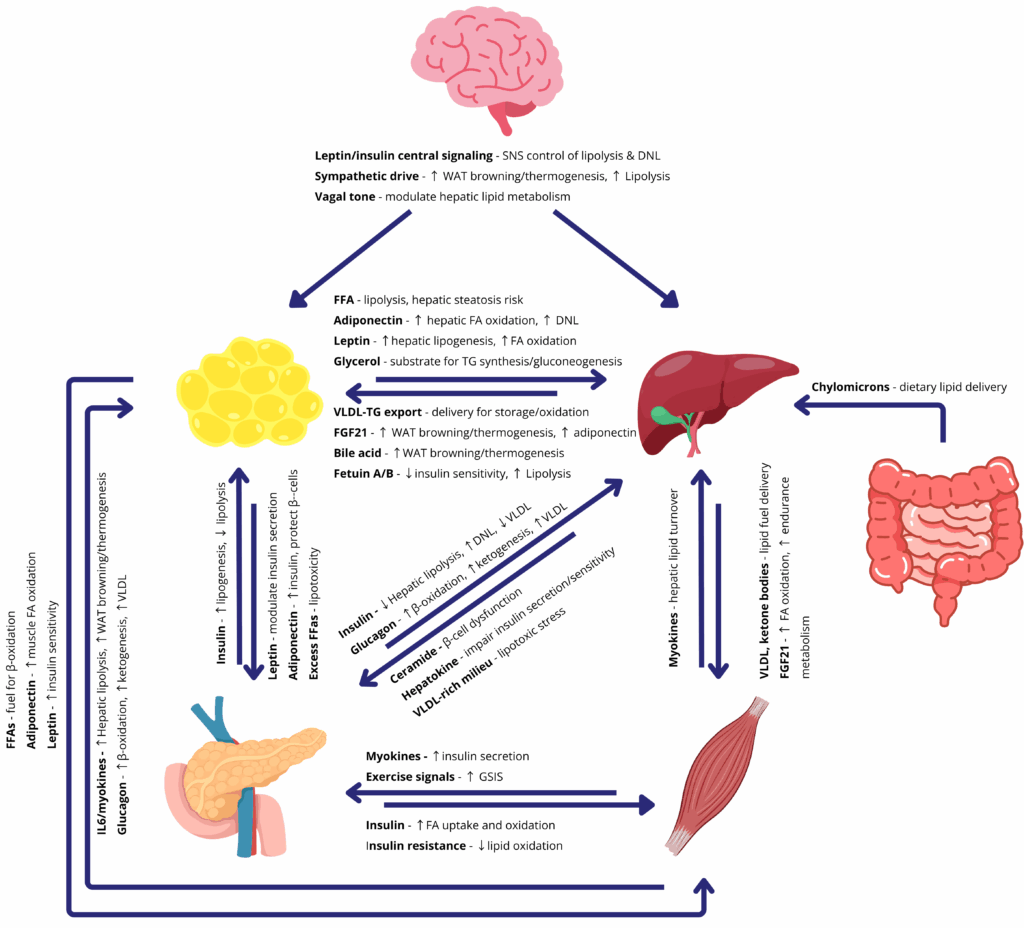
Key Organs of Lipid Metabolism and Their Crosstalk in Disease
The body’s major metabolic organs, adipose tissue (fat), the liver, skeletal muscle, and the pancreas, each play distinct roles in lipid metabolism and energy balance. These organs are in constant communication via hormones, nutrients, and nerves to maintain homeostasis. When this multi-organ crosstalk functions properly, it enhances fat burning, limits fat accumulation, and keeps blood lipid levels in check. Conversely, when the network breaks down, as in obesity, the resulting imbalances lead to metabolic pathologies.
Adipose tissue is the primary energy reservoir, storing excess calories as triglycerides. In times of plenty, white adipose tissue expands to safely sequester fat and prevent lipids from flooding other organs. Far from being inert storage, fat is also an active endocrine organ: it releases signaling molecules called adipokines (like leptin and adiponectin) that help regulate appetite, insulin sensitivity, and inflammation. Under healthy conditions, leptin from fat signals the brain to reduce hunger and helps muscles and liver adjust fuel usage, while adiponectin boosts fatty acid oxidation in muscle and curbs glucose production in the liver. These hormonal signals ensure that lipids are stored and utilized efficiently. In obesity, however, adipose signaling is disrupted, chronically high leptin levels lead to leptin resistance (the brain and body stop responding), and adiponectin levels plummet. This results in unchecked appetite, reduced fat-burning in muscles, and higher fat buildup in the liver. The dysfunction of fat tissue thus directly contributes to conditions like insulin resistance and fatty liver disease. Indeed, excessive fat accumulation in the liver defines NAFLD, and this can progress to an inflammatory state called non-alcoholic steatohepatitis (NASH) with fibrosis (scarring of the liver) if metabolic stress persists.
The liver is a central hub of lipid metabolism. It manufactures, stores, and exports fats (e.g. cholesterol and triglycerides) and helps distribute energy throughout the body. The liver takes up free fatty acids from the circulation, for instance, after a high-fat meal or when released by adipose tissue during fasting. If the influx of fat overwhelms the liver’s capacity, fat begins to accumulate in liver cells (hepatocytes). This is the hallmark of non-alcoholic fatty liver disease (NAFLD), a condition now common in individuals with obesity and metabolic syndrome. NAFLD can further induce inflammation and damage (NASH), potentially progressing to liver fibrosis or cirrhosis. The liver also communicates with other organs by secreting its own hormones and factors. For example, it produces fibroblast growth factor 21 (FGF21) in response to metabolic stress, which can promote fat oxidation in adipose tissue and muscle. In metabolic disease, these inter-organ feedback loops are disrupted, an overburdened fatty liver not only fails to regulate blood lipids properly, but also sends out abnormal metabolic signals that can exacerbate whole-body insulin resistance and dyslipidemia.
Skeletal muscle plays a pivotal role as a consumer of lipids. Muscle fibers use fatty acids as a key fuel, especially during physical activity, and healthy muscle tissue helps clear fats and sugars from the bloodstream under insulin’s direction. There is a two-way conversation between muscle and fat. On one hand, adipose-derived hormones like leptin and adiponectin enhance muscle’s ability to oxidize fatty acids and take up glucose, keeping muscle insulin-sensitive. On the other hand, working muscles release myokines (muscle-secreted factors) that influence fat tissue. During exercise, for instance, muscles secrete molecules such as IL-6 and irisin that travel through the blood to adipose depots, prompting increased fat breakdown and the “browning” of white fat (i.e. white fat cells taking on features of heat-producing brown fat). This muscle-to-fat crosstalk boosts energy expenditure and can counteract obesity-related weight gain. In sedentary or obese states, however, muscle can become a victim of lipid overload: fat droplets accumulate within muscle fibers when caloric intake is chronically high, leading to insulin resistance in muscle. As muscles become less responsive to insulin, blood glucose levels rise, forcing the pancreas to work harder. The pancreas, through its insulin-producing β-cells, attempts to compensate for insulin resistance by secreting more insulin. Over time, this strain can cause β-cell dysfunction or failure, precipitating type 2 diabetes. Thus, ectopic fat in muscle and other tissues links obesity to diabetes by blunting insulin’s effects and overwhelming the pancreas.
Other organs and systems join this metabolic web as well. The brain (particularly the hypothalamus) senses adipose signals like leptin to control appetite and energy expenditure; when leptin resistance develops, the brain fails to curb appetite, contributing to further weight gain. The intestine is the gateway for dietary lipids, packaging fats into chylomicrons that are delivered to adipose tissue and liver. Gut-derived hormones (such as GLP-1) and even the gut microbiome can influence liver fat accumulation and systemic metabolism, forming a gut–liver axis implicated in NAFLD. Additionally, chronic inflammation arising from enlarged adipose depots in obesity acts as a common thread disrupting metabolic signaling. Pro-inflammatory cytokines (secreted by immune cells in fat and liver) can worsen insulin resistance and organ crosstalk, creating a vicious cycle of metabolic deterioration.
In summary, metabolic diseases are multi-organ in nature: an imbalance in one organ reverberates through others. Obesity illustrates this clearly: excessive adipose tissue alters hormone levels and free fatty acid release, which in turn fatten the liver, tax the pancreas, and reduce muscle insulin sensitivity, ultimately driving conditions like NAFLD and type 2 diabetes. Conversely, when the liver, fat, and muscle communicate properly, the body can efficiently handle lipids and maintain metabolic health(1,2). This intricate physiology is why researchers are keen to study organ crosstalk in the context of disease. Traditional cell cultures or animal models often fail to capture the human-specific interactions between, say, human liver and human fat tissue. As a result, scientists are turning to advanced in vitro systems to model these relationships. Organoids (3D mini-organs grown from stem cells) and organ-on-a-chip platforms (microfluidic devices simulating organ function) now make it possible to investigate human organ interactions under controlled conditions. In the following sections, we will see how single-organ models – including adipose tissue organoids and liver organoids on chips – are shedding light on metabolic disease mechanisms, and how multi-organ microphysiological models are being used to recreate the complex cross-talk of metabolic organs in the lab(3).
Organoids and Organ-on-Chip Models for Studying Lipid Metabolism
Organoids and organ-on-chip systems are powerful approaches to recreate human metabolism in vitro. Organoids, grown from stem or primary cells, self-organize into 3D structures that preserve tissue-specific cell types and metabolic programs, such as lipid storage in adipocytes or glucose release in hepatocytes. Organ-on-chip devices combine living cells with microfluidics to mimic blood flow, nutrient delivery, and tissue–tissue interfaces, enabling real-time readouts of lipid uptake, secretion, and hormonal signals. Their design overcomes many limitations of traditional cultures by allowing chronic exposure studies, spatial organization, and non-invasive sampling, making them particularly relevant for metabolic disease research.
Adipose tissue
Adipose tissue stores energy as triglycerides and releases free fatty acids during fasting, while also secreting adipokines that regulate appetite, insulin sensitivity, and inflammation. Dysregulated adipose function in obesity alters lipid release and systemic metabolism. To capture these processes, adipose-on-chip models culture human mature adipocytes under flow, maintaining their viability and functionality. For instance, Rogal et al. developed a white adipose tissue-on-chip that enabled real-time imaging of fatty acid uptake and quantification of glycerol and free fatty acid release in effluents, demonstrating both storage and mobilization functions of human fat tissue under controlled conditions(4). Similarly, Huff et al. optimized a fat-on-a-chip model for non-invasive monitoring, showing how adipocytes equilibrate droplet size within extracellular matrices and respond to insulin by increasing glucose uptake(5). These models illustrate how adipose tissue’s role in energy storage and release can be probed directly in human-derived platforms.
Liver
The liver orchestrates lipid synthesis, oxidation, and export, and is the central organ affected in non-alcoholic fatty liver disease (NAFLD). Liver-on-chip and liver organoid models replicate features of the sinusoid, cell diversity, and metabolic gradients. Freag et al. built a NASH-on-a-chip with hepatocytes, Kupffer, stellate, and endothelial cells, which developed hallmark features of disease (steatosis, inflammation, and fibrosis) under lipid overload, and responded to pharmacological treatment with reduced lipid accumulation(6). Gori et al. showed that microfluidic perfusion of fatty acids in a liver-on-chip sinusoid model resulted in gradual lipid accumulation with better cell viability than static cultures, more closely mirroring chronic steatosis(7). More recently, Igarashi et al. generated human hepatocyte organoids capable of long-term expansion and adult metabolic functions, including bile canalicular networks and zonated lipid metabolism, representing a powerful tool for studying both physiology and pathology(8).
Pancreas
Pancreatic β-cells secrete insulin, the hormone that regulates lipid and glucose metabolism across tissues. Their dysfunction under lipid stress is central to type 2 diabetes. Organoid and chip systems provide ways to study this process with high temporal resolution. Dishinger et al. created a microfluidic chip to continuously measure insulin secretion from single islets, demonstrating that free fatty acid exposure abolished pulsatile insulin release, a key marker of β-cell dysfunction(9). Bandak et al. similarly showed that while saturated fatty acids impair insulin oscillations, beneficial lipid species such as FAHFAs improve insulin secretion patterns in microfluidic assays(10). These findings underscore how organ-on-chip technologies reveal the direct influence of lipid stress on pancreatic function.
Skeletal muscle
Skeletal muscle consumes large amounts of fatty acids during activity and is crucial for maintaining insulin sensitivity. Muscle-on-chip systems reproduce human myofiber alignment, contractility, and metabolism under controlled environments. Kim et al. reported a skeletal muscle-on-chip that, when tested in microgravity, showed a metabolic shift toward fatty acid use but impaired regeneration capacity. Treatment with candidate drugs partially restored muscle function, highlighting the platform’s potential to screen interventions for lipid-related muscle dysfunction(11).
Intestine
The intestine is the site of dietary lipid absorption and chylomicron production, critical for delivering fats to adipose tissue and liver. Intestine-on-chip models recreate villus-like structures, barrier function, and flow conditions. Bein et al. used an intestine-on-a-chip with patient-derived cells to model environmental enteric dysfunction, showing that nutrient deficiency caused villus atrophy, barrier breakdown, and impaired fatty acid uptake, closely resembling clinical pathology(12). This demonstrates how gut-on-chip platforms can shed light on how dietary and environmental factors shape lipid handling.
For further reading
A Multi-Organ Perspective: How Organ-on-Chip Systems Reveal Disease Mechanisms
Metabolic diseases like non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D) are systemic conditions, not isolated organ failures. They arise from complex and dysfunctional communication between multiple tissues. Traditional in vitro 2D cell cultures and even animal models often fail to capture this intricate inter-organ dialogue, limiting our understanding and the development of effective therapies. To truly decipher these diseases, we must study organ crosstalk. Advanced organoids for metabolic disease research is meeting this challenge by using microfluidic organ-on-chip platforms. These systems connect different tissues, such as liver organoids and adipose tissue organoids, in a single, dynamic device, allowing scientists to observe how these tissues communicate in real-time and providing insights that are impossible to obtain from studying organs in isolation.
The Gut-Adipose-Liver Axis
The dialogue between fat, the gut, and the liver is central to NAFLD. In one pivotal study, an adipose-liver human-on-a-chip model was essential to demonstrate how inflammation drives liver steatosis. Researchers found that when adipose tissue was included, the inflammatory signal TNF-α induced significant fat accumulation (steatosis) in the liver cells—an effect that was completely absent when liver cells were cultured alone. The platform enabled the monitoring of altered adipokine secretion, such as adiponectin, revealing how dysfunctional adipose tissue directly impacts liver health(15). Similarly, an integrated gut-liver-on-a-chip platform revealed a protective crosstalk; co-cultured gut and liver cells were shielded from apoptosis when treated with high levels of free fatty acids, a protective effect not observed in monocultures. This underscores that signals from surrounding metabolic organs are required to initiate and modulate key features of liver disease(16).
Pancreatic Crosstalk with Liver and Muscle
The interplay between the pancreas and other organs is crucial for glucose control. An organ-on-chip system that functionally coupled liver organoids (spheroids) and pancreatic islets was necessary to reconstruct a physiological feedback loop. Insulin secreted from the islets in response to glucose stimulated uptake by the liver, which in turn lowered glucose levels and modulated subsequent insulin secretion—a dynamic, self-regulating system that cannot be replicated in single-organ cultures(17).
Another study using a connected liver-pancreas system demonstrated that a liver model mimicking early metabolic syndrome could induce pancreatic islet dysfunction only when the two systems were coupled. The standalone pancreatic model showed no significant dysfunction under the same media conditions, proving that specific hepatokines (like Fetuin A) secreted by the diseased liver were directly responsible for impairing the pancreas(18).
Beyond the liver, muscle tissue also communicates with the pancreas. A “Training-on-a-Chip” featuring functional, contracting 3D muscle tissue was required to show that electrical stimulation mimicking exercise causes the release of myokines (like IL-6) that directly trigger insulin secretion from pancreatic cells. This provided direct evidence for the beneficial exercise-pancreas link, an interaction that could only be confirmed by studying the two organs in communication(19).
Modeling Systemic Disease and Complications
Systemic diseases like T2D involve even more complex crosstalk. A triple organ-on-chip model incorporating the pancreas, liver, and adipose tissue organoids was necessary to fully recapitulate the key pathological features of T2D and its complications. Using 3D printing with tissue-specific bioinks, this platform showed that disease characteristics only appeared when all three organs could interact within a hyperglycemic environment. This model was so accurate that it could predict the clinical efficacy of T2D medications(20).
This multi-organ crosstalk also extends to other diseases, such as cancer. For instance, co-culture models using a 3D fibrin matrix were essential to keep primary adipocytes viable to show that adipose tissue organoids from obese individuals amplify the transfer of fatty acids to breast cancer cells, potentially fueling tumor progression(21). Other work has shown that factors from both lean and obese adipose tissue can induce a partial mesenchymal-to-epithelial transition in cancer cells, giving them hybrid properties associated with more aggressive tumors(22).
These examples powerfully illustrate that microfluidic organ-on-chip systems are a necessity for modern metabolic research. By connecting different organoids, these platforms allow us to decipher the intricate conversations between organs that drive health and disease. It is this essential crosstalk, revealed by technologies like liver organoids and adipose tissue organoids on a chip, that holds the key to developing personalized therapies and ultimately conquering complex metabolic diseases.
Conclusion & Future Directions
In summary, organoids for lipid metabolism and organ-on-chip models offer transformative platforms to explore how key organs like the liver, adipose tissue, pancreas, muscle, and intestine manage fats in health and disease. These technologies enable precise modeling of lipid uptake, hormonal interplay, and metabolic dysfunctions underlying obesity, NAFLD, and diabetes.
Looking ahead, the next leap forward lies in multi‐tissue integration, combining vascularized, immune-enabled, and perfused organoids on-chip, to better mimic systemic lipid trafficking and inter-organ crosstalk. Such advances promise to accelerate metabolic disease modeling and drug discovery, bridging human physiology and predictive preclinical testing.
References
- Zhu Y, Wan F, Liu J, Jia Z, Song T. The Critical Role of Lipid Metabolism in Health and Diseases. Nutrients. 23 déc 2024;16(24):4414.
- Jia Z, Wang Z, Pan H, Zhang J, Wang Q, Zhou C, et al. Crosstalk between fat tissue and muscle, brain, liver, and heart in obesity: cellular and molecular perspectives. Eur J Med Res. 31 déc 2024;29(1):637.
- Liu X, Yang J, Yan Y, Li Q, Huang RL. Unleashing the potential of adipose organoids: A revolutionary approach to combat obesity-related metabolic diseases. Theranostics. 2024;14(5):2075‑98.
- Rogal J, Binder C, Kromidas E, Roosz J, Probst C, Schneider S, et al. WAT-on-a-chip integrating human mature white adipocytes for mechanistic research and pharmaceutical applications. Sci Rep. 20 avr 2020;10(1):6666.
- Huff LK, Amurgis CM, Kokai LE, Abbott RD. Optimization and validation of a fat-on-a-chip model for non-invasive therapeutic drug discovery. Front Bioeng Biotechnol. 25 juin 2024;12:1404327.
- Freag MS, Namgung B, Reyna Fernandez ME, Gherardi E, Sengupta S, Jang HL. Human Nonalcoholic Steatohepatitis on a Chip. Hepatol Commun. févr 2021;5(2):217‑33.
- Gori M, Simonelli MC, Giannitelli SM, Businaro L, Trombetta M, Rainer A. Investigating Nonalcoholic Fatty Liver Disease in a Liver-on-a-Chip Microfluidic Device. Gracia-Sancho J, éditeur. PLOS ONE. 20 juill 2016;11(7):e0159729.
- Igarashi R, Oda M, Okada R, Yano T, Takahashi S, Pastuhov S, et al. Generation of human adult hepatocyte organoids with metabolic functions. Nature [Internet]. 16 avr 2025 [cité 13 mai 2025]; Disponible sur: https://www.nature.com/articles/s41586-025-08861-y
- Dishinger JF, Reid KR, Kennedy RT. Quantitative Monitoring of Insulin Secretion from Single Islets of Langerhans in Parallel on a Microfluidic Chip. Anal Chem. 15 avr 2009;81(8):3119‑27.
- Bandak B, Yi L, Roper MG. Microfluidic-enabled quantitative measurements of insulin release dynamics from single islets of Langerhans in response to 5-palmitic acid hydroxy stearic acid. Lab Chip. 2018;18(18):2873‑82.
- Kim S, Ayan B, Shayan M, Rando TA, Huang NF. Skeletal muscle-on-a-chip in microgravity as a platform for regeneration modeling and drug screening. Stem Cell Rep. août 2024;19(8):1061‑73.
- Bein A, Fadel CW, Swenor B, Cao W, Powers RK, Camacho DM, et al. Nutritional deficiency in an intestine-on-a-chip recapitulates injury hallmarks associated with environmental enteric dysfunction. Nat Biomed Eng. 23 juin 2022;6(11):1236‑47.
- McCarthy M, Brown T, Alarcon A, Williams C, Wu X, Abbott RD, et al. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng Part B Rev. 1 déc 2020;26(6):586‑95.
- Moradi E, Jalili-Firoozinezhad S, Solati-Hashjin M. Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater. oct 2020;116:67‑83.
- Slaughter VL, Rumsey JW, Boone R, Malik D, Cai Y, Sriram NN, et al. Validation of an adipose-liver human-on-a-chip model of NAFLD for preclinical therapeutic efficacy evaluation. Sci Rep. 23 juin 2021;11(1):13159.
- Yang J, Hirai Y, Iida K, Ito S, Trumm M, Terada S, et al. Integrated-gut-liver-on-a-chip platform as an in vitro human model of non-alcoholic fatty liver disease. Commun Biol. 23 mars 2023;6(1):310.
- Bauer S, Wennberg Huldt C, Kanebratt KP, Durieux I, Gunne D, Andersson S, et al. Functional coupling of human pancreatic islets and liver spheroids on-a-chip: Towards a novel human ex vivo type 2 diabetes model. Sci Rep. 3 nov 2017;7(1):14620.
- Aleman J, K R, Wiegand C, Schurdak ME, Vernetti L, Gavlock D, et al. A metabolic dysfunction-associated steatotic liver acinus biomimetic induces pancreatic islet dysfunction in a coupled microphysiology system. Commun Biol. 14 oct 2024;7(1):1317.
- Fernández‐Costa JM, Ortega MA, Rodríguez‐Comas J, Lopez‐Muñoz G, Yeste J, Mangas‐Florencio L, et al. Training‐on‐a‐Chip: A Multi‐Organ Device to Study the Effect of Muscle Exercise on Insulin Secretion in Vitro. Adv Mater Technol. avr 2023;8(7):2200873.
- Kim JJ, Park JY, Nguyen VVT, Bae M, Kim M, Jang J, et al. Pathophysiological Reconstruction of a Tissue-Specific Multiple-Organ On-A-Chip for Type 2 Diabetes Emulation using 3D Cell Printing. Adv Funct Mater. 1 mai 2023;33(22):2213649.
- Rebeaud M, Bouche C, Dauvillier S, Attané C, Arellano C, Vaysse C, et al. A novel 3D culture model for human primary mammary adipocytes to study their metabolic crosstalk with breast cancer in lean and obese conditions. Sci Rep. 22 mars 2023;13(1):4707.
- Asante EC, Pallegar NK, Hoffmann AJ, Viloria-Petit AM, Christian SL. Adipose Tissue from Lean and Obese Mice Induces a Mesenchymal to Epithelial Transition-Like Effect in Triple Negative Breast Cancers Cells Grown in 3-Dimensional Culture. Int J Mol Sci. 3 sept 2020;21(17):6439.
FAQ
Traditional models are often found to be insufficient. They fail to capture the intricate nature of human lipid metabolism. The processing of fats in the body is understood to be dependent on the coordinated activity of several organs. These include the liver, adipose tissue, the pancreas, and muscle. Capturing the way these organs communicate is not done well by older systems. This failure makes it difficult to study conditions like obesity, type 2 diabetes, and fatty liver disease. New technologies, such as organoids and organ-on-chip platforms, are now being used. These systems make it possible to recreate these metabolic processes in a laboratory setting.
Adipose tissue is the body’s main energy reservoir. Excess calories are stored as triglycerides within this tissue. White adipose tissue expands when needed. This expansion safely sequesters fat and prevents lipids from flooding other organs. Adipose tissue is also an active endocrine organ. Signaling molecules called adipokines (like leptin and adiponectin) are released. These signals help regulate appetite, insulin sensitivity, and inflammation. Under healthy conditions, these hormones ensure lipids are stored and used efficiently. When obesity occurs, this adipose signaling is disrupted. Leptin resistance can develop and adiponectin levels may fall. This dysfunction contributes to insulin resistance.
The liver is described as a central hub for lipid metabolism. Fats, such as cholesterol and triglycerides, are manufactured, stored, and exported by the liver. Energy is distributed throughout the body by this organ. Free fatty acids are taken up from the circulation. This occurs after a high-fat meal or when released by adipose tissue. If the influx of fat overwhelms the liver’s capacity, accumulation begins in liver cells (hepatocytes). This condition is the defining feature of non-alcoholic fatty liver disease (NAFLD). This state can progress to include inflammation and damage (NASH). Liver fibrosis may eventually occur if the metabolic stress continues.
Skeletal muscle is a consumer of lipids. Fatty acids are used by muscle fibres as a fuel, especially during physical activity. Healthy muscle tissue assists in clearing fats and sugars from the bloodstream, a process directed by insulin. A two-way conversation exists between muscle and fat. Myokines are factors secreted by working muscles. These molecules travel to adipose depots. Increased fat breakdown is prompted by their arrival. In sedentary states, muscle can be affected by lipid overload. Fat droplets accumulate within muscle fibres. This accumulation leads to insulin resistance in the muscle. Blood glucose levels then rise, forcing the pancreas to work harder.
The body’s major metabolic organs are in constant communication. This dialogue occurs via hormones, nutrients, and nerves. Homeostasis is maintained by this system. When this multi-organ network breaks down, such as in obesity, the resulting imbalances lead to metabolic pathologies. Metabolic diseases are considered multi-organ in nature. An imbalance in one organ is felt by other organs. Obesity provides a clear illustration of this. Excessive adipose tissue alters hormone levels. It also changes free fatty acid release. This change in turn can lead to a fatty liver, a taxed pancreas, and reduced insulin sensitivity in muscle. These conditions, like NAFLD and type 2 diabetes, are driven by this breakdown in communication.
Adipose-on-chip models are systems where human mature adipocytes are cultured under fluid flow. This method is used to maintain their viability and functionality. One such model of white adipose tissue was developed. It permitted real-time imaging of fatty acid uptake. Quantification of glycerol and free fatty acid release into the effluents was also made possible. This demonstrated both the storage and mobilization functions of human fat tissue under controlled conditions. Another model was refined for non-invasive monitoring. It showed how adipocytes equilibrate droplet size and respond to insulin by increasing glucose uptake.
Liver-on-chip and liver organoid models are used to reproduce features of the liver. These include the sinusoid, cell diversity, and metabolic gradients. One specific NASH-on-a-chip was built. It contained hepatocytes, Kupffer, stellate, and endothelial cells. When subjected to lipid overload, this model developed the hallmark features of the disease. These features included steatosis, inflammation, and fibrosis. The model also responded to pharmacological treatment, showing reduced lipid accumulation. Other researchers have generated human hepatocyte organoids. These are capable of long-term expansion. They also maintain adult metabolic functions. This includes the formation of bile canalicular networks and the presence of zonated lipid metabolism. These systems are considered useful for studying physiology and pathology.
The dysfunction of pancreatic β-cells under lipid stress is a central component of type 2 diabetes. Organoid and chip systems are used to study this process. One microfluidic chip was created. It was used to continuously measure insulin secretion from single islets. Through this system, it was demonstrated that exposure to free fatty acids abolished the pulsatile release of insulin. This loss of pulsatility is known to be a marker of β-cell dysfunction. Other work has shown that saturated fatty acids impair insulin oscillations. In contrast, beneficial lipid species were found to improve insulin secretion patterns in these microfluidic assays.
Muscle tissue is known to communicate with the pancreas. A “Training-on-a-Chip” was developed. This chip featured functional, contracting 3D muscle tissue. Electrical stimulation was applied to the muscle. This stimulation was intended to reproduce the effects of exercise. It was shown that this action caused the release of myokines, including IL-6. These molecules were then observed to travel and directly trigger the secretion of insulin from pancreatic cells. Direct evidence for the beneficial connection between exercise and the pancreas was provided by this experiment. This interaction could only be confirmed by studying the two organs in communication.