In this discussion, we investigate the optimal cell source for constructing 3D adipose models, predicated on the specific research objective. A primary consideration is whether the experimental aim requires clean, comparable readouts for metabolic processes such as lipid handling and insulin signaling, or alternatively, necessitates the inclusion of stromal crosstalk, capillary networks, and innate immune components within the microtissue. The selection between the stromal vascular fraction (SVF) and adipose-derived stromal cells (ADSC) fundamentally dictates the subsequent experimental outcomes. Herein, we delineate the definitions, isolation methodologies, and comparative advantages and limitations of each source, concluding with specific SVF vs ADSC organoid model examples.
Learn more about our ready to use Adipose organoid plate.
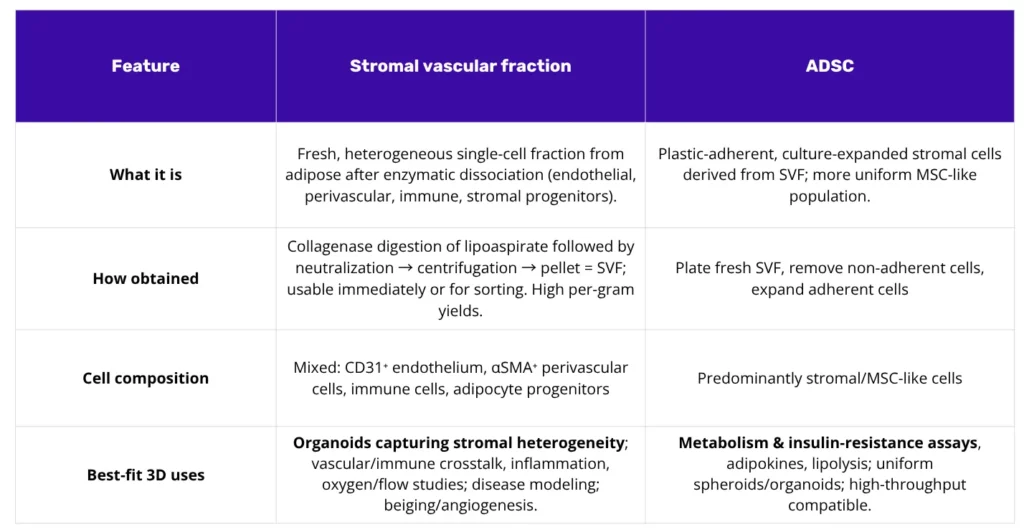
Definitions and isolation methodologies for SVF and ADSC
Stromal Vascular Fraction (SVF)
SVF, the stromal vascular fraction, represents the heterogeneous single-cell pellet released from adipose tissue following enzymatic collagenase digestion. It is not a singular cell type, but rather a complex population. This mixture includes endothelial cells, pericytes, adipocyte progenitors, fibroblasts, mast cells, macrophages, and various other stromal partners. For flow cytometry, the stromal progenitor gate is typically enriched via the exclusion of hematopoietic and endothelial markers (CD45⁻, CD235a⁻, CD31⁻) and the selection of CD34⁺ cells, often validated against MSC-associated antigens (e.g., CD73, CD90, CD105)(1). Recently, some adipose-resident pro-preadipocyte have also been identified as ICAM1+ CD44high Noct-expressing cells, highlighting once again the heterogeneity of this tissue fraction(2).
The isolation protocol involves mincing lipoaspirate or surgical fat, followed by collagenase digestion, neutralization, and centrifugation. Mature adipocytes become buoyant, while the cell pellet at the bottom constitutes the SVF. Reports have long noted high progenitor yields per gram of tissue, establishing adipose tissue as a favored source for cellular applications.
Adipose-Derived Stromal Cells (ADSC)
ADSCs (alternatively ASCs) are functionally distinct. They are defined as the plastic-adherent, culture-expanded stromal cell population derived from SVF. The methodology entails plating the fresh SVF, removing non-adherent cells via rinsing, and subsequently expanding the adherent, spindle-shaped population. Following several passages, a reproducible, MSC-like immunophenotype emerges: characterized by positivity for CD13, CD29, CD44, CD73, CD90, and CD105, while remaining negative for hematopoietic and endothelial markers. A useful distinction is CD34 positivity at early passages, which wanes with time in culture. This population serves as a consistent, bankable cell source for engineered 3D systems(3).
Comparative analysis of SVF and ADSC in 3D culture
The impact of SVF on microenvironment study
The heterogeneous composition of SVF facilitates self-organization within 3D constructs. This mixture enables the formation of endothelial strands, which are subsequently wrapped by perivascular cells, while resident immune cells contribute to the cytokine milieu. Adipocyte progenitors then differentiate within this shared microenvironment. Consequently, if the research objective involves vascular remodeling, chronic low-grade inflammation, oxygen tension, or emergent multicellular behaviors, SVF provides a more comprehensive starting population(4).
SVF is well-suited for organoid models that aim to preserve this heterogeneity by design. This approach is advantageous for complex disease modeling where the endogenous niche is required to contribute to the phenotype, rather than reconstructing each cellular component individually(5).
The role of ADSC in standardization and scale
ADSCs, by contrast, are preferable when study parameters prioritize standardization, repeatability, and scalability. This population can be readily expanded, cryopreserved, utilized in multi-donor panels, and used to generate spheroids or hydrogel constructs with high uniformity. If the primary endpoints are quantitative metabolic metrics, such as lipid accumulation, adipokine secretion, lipolysis, or insulin signaling, ADSCs represent the more practical selection for achieving comparable data across numerous conditions. They also integrate predictably into microphysiological systems and decellularized adipose hydrogels.
Methodological advantages and limitations
SVF: Strengths and considerations
The defining advantage of SVF is its native heterogeneity. This is particularly beneficial for modeling insulin resistance driven by inflammatory cues, endothelial–adipocyte dialogue, or ECM–vascular co-adaptation under flow. Many labs now employ SVF for organoid generation precisely to preserve these interactions. However, significant limitations exist: donor variability is often wider, the presence of immune components complicates allogeneic applications, and the enzymatic isolation protocol may be subject to stricter regulatory categories, unless closed devices or mechanical alternatives (which may sacrifice yield) are utilized.
ADSC: Strengths and considerations
The primary advantages of ADSC are standardization and logistical ease. They demonstrate reliable expansion, are amenable to cryobanking, and integrate into engineered matrices with predictable attachment and growth. The limitations relate to biological fidelity: extended culture can induce phenotypic shifts, and monocultures inherently under-represent the immune and endothelial compartments unless these populations are exogenously added.
A critical consideration for both sources is that achieving mature, long-lived unilocular adipocytes in vitro remains a significant challenge. While advances in biomaterials, human decellularized adipose hydrogels, and microfluidics are closing this gap, fully native behavior often appears only when the stromal cast is present and oxygen delivery is optimized. This challenge explains why many research programs utilize SVF organoids for initial discovery, followed by quantification using precisely defined ADSC-based constructs(6).
Application-specific model selection
Models for metabolism and insulin signaling readouts
Conversely, if the biological signal is dependent on intercellular crosstalk (for instance involving capillaries, perivascular cells, or resident immune cells) SVF is the appropriate starting material. The resultant organoids preserve these cellular interactions. This is the preferred route for models of inflammation-modulated insulin resistance, angiogenesis and barrier function, or investigations into how oxygen supply dictates lipid storage and survival under stress. Perfusion systems are a natural application for these complex models(5).
An integrative approach: From discovery to measurement
One pattern gaining traction is a two-step approach: discovery with SVF, followed by measurement with ADSC. An SVF organoid is first employed to capture the complex phenomenon and identify the key cellular players. Once the critical mechanisms are known, a minimal system can be reconstructed using ADSC plus the specific endothelial or immune partners required for a more controlled, higher-throughput screen. Reviews of engineered adipose platforms increasingly point to this strategy(6).
Together, breast cancer-on-chip technologies complement cell-line and patient-derived organoids by introducing dynamic control and physiological fluid exchange. They support quantitative analysis of drug transport, immune engagement, and microenvironmental adaptation under human-relevant conditions. As standardization advances, these microengineered models are expected to become integral components of preclinical pipelines, refining predictions of therapeutic performance and resistance in HER2-positive breast cancer.
Exemples of studies and methodologies
ADSC models for metabolism and screening
Several studies provide frameworks for ADSC-based models. A widely cited organoid model from Mandl and colleagues utilizes a scaffold-free pipeline (hanging drops followed by agar support) to grow human ASC. These organoids differentiate, accumulate large lipid droplets, and secrete adiponectin at levels sufficient for endocrine endpoint analysis. The system’s uniformity lends itself to time-course studies, donor comparisons, and compound testing(7).
Klingelhutz and co-workers described uniform adipose spheroids that mature over approximately one month and outperform monolayers on key functions, such as adiponectin production. This format reacts to toxic stress and inflammatory stimuli, providing a compact test bed for metabolic toxicity and adipokine modulation(8).
SVF organoids preserving tissue architecture
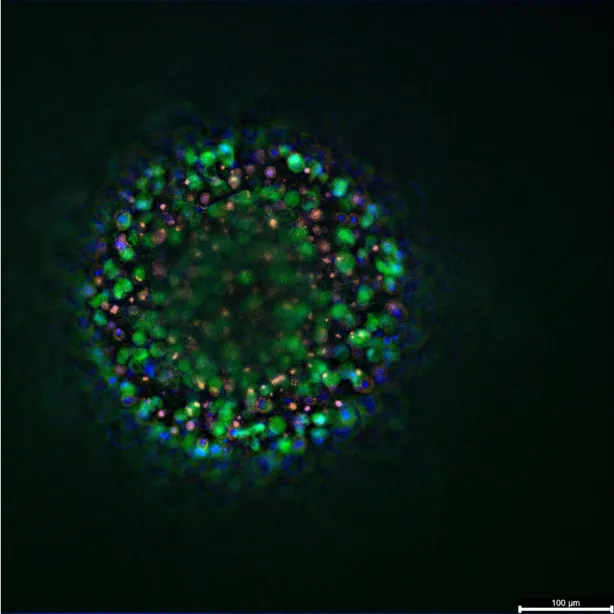
For SVF-based models, Muller and colleagues demonstrated the growth of 3D adipose tissues from human SVF, which developed visible CD31⁺ vascular strands and αSMA⁺ perivascular coverage that persisted through adipogenesis. The adipocytes achieved a unilocular state while microvessels remained. This platform is suited for questions involving vascular remodeling and adipocyte–endothelium dialogue(9).
Robledo and co-authors utilized neonatal brown adipose SVF to produce complex spheroids that stratified, with mature adipocytes at the periphery and progenitors toward the core. The model showed robust endocrine behavior and an adrenergic lipolysis response superior to monolayer cultures, making it a clear reference for studies of endocrine function in a 3D context(10).
Furthermore, perfusion impacts these models. In our microphysiological setup at Cherry Biotech, we observed that human SVF organoids grown under flow show stronger lipid accumulation, lower necrosis, and preservation of mixed populations including CD31⁺ cells. We demonstrated that cryopreserved SVF remains competent to form mature unilocular adipocytes, aiding planning and logistics. We have also maintained adipose tissue slices under high oxygen, keeping droplets and capillaries intact for one week, as a method to probe oxygen and flow effects. This work is detailed further in our recent poster presentation you can check it out here !
Conclusion
Our recommendation for cell source is framed by the primary readouts and biological complexity required.
If the endpoints are quantitative and metabolism-centric (lipid content, adipokines, insulin response) and the plan includes multi-condition screens, ADSC models provide cleaner comparisons. They offer size-controlled spheroids, consistent integration into hydrogels, and a bankable cell source. Defined endothelial or immune partners can be added exogenously to layer specific interactions while maintaining control(5).
If the biological signal is contingent on the native stromal microenvironment (endothelium, perivascular support, resident immune cells) SVF is the recommended choice. The organoids that emerge preserve these critical interactions. This is the route for models of inflammation-driven insulin resistance, angiogenesis, barrier function, or exploring how oxygen supply dictates lipid storage. Perfusion systems tend to amplify these differences(5).
Conclusion
SVF and ADSC should not be viewed as rival methodologies, but rather as distinct tools for different experimental objectives. When the research goal requires neighborhood dynamics, we select SVF to allow the organoid to self-assemble. When the objective is scalability and clean quantitative comparisons, we select ADSC to build the microenvironment with precision. In practice, many programs utilize both: SVF for discovery of complex phenomena, and ADSC to measure the key mechanisms with high control. As detailed on our poster, our work demonstrates how SVF performs within a microphysiological platform, which is a key consideration for studies where flow and oxygen are part of the experimental design.
References
- Brown JC, Katz AJ. Stem Cells Derived From Fat. In: Principles of Regenerative Medicine [Internet]. Elsevier; 2019 [cité 5 nov 2025]. p. 295‑305. Disponible sur: https://linkinghub.elsevier.com/retrieve/pii/B9780128098806000199
- Chen M, Kim S, Li L, Chattopadhyay S, Rando TA, Feldman BJ. Identification of an adipose tissue-resident pro-preadipocyte population. Cell Rep. mai 2023;42(5):112440.
- Noël D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, et al. Cell specific differences between human adipose-derived and mesenchymal–stromal cells despite similar differentiation potentials. Exp Cell Res. avr 2008;314(7):1575‑84.
- Bora P, Majumdar AS. Adipose tissue-derived stromal vascular fraction in regenerative medicine: a brief review on biology and translation. Stem Cell Res Ther. déc 2017;8(1):145.
- Navarro-Perez J, Carobbio S. Adipose tissue-derived stem cells, in vivo and in vitro models for metabolic diseases. Biochem Pharmacol. avr 2024;222:116108.
- Yoon H, Park TE. Engineered adipose tissue platforms: recent breakthroughs and future perspectives. Organoid. 25 janv 2023;3:e1.
- Mandl M, Viertler HP, Hatzmann FM, Brucker C, Großmann S, Waldegger P, et al. An organoid model derived from human adipose stem/progenitor cells to study adipose tissue physiology. Adipocyte. 31 déc 2022;11(1):164‑74.
- Klingelhutz AJ, Gourronc FA, Chaly A, Wadkins DA, Burand AJ, Markan KR, et al. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci Rep. 11 janv 2018;8(1):523.
- Muller S, Ader I, Creff J, Leménager H, Achard P, Casteilla L, et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Sci Rep. 10 mai 2019;9(1):7250.
- Robledo F, González-Hodar L, Tapia P, Figueroa AM, Ezquer F, Cortés V. Spheroids derived from the stromal vascular fraction of adipose tissue self-organize in complex adipose organoids and secrete leptin. Stem Cell Res Ther. 7 avr 2023;14(1):70.
FAQ
The selection between the stromal vascular fraction (SVF) and adipose-derived stromal cells (ADSC) is dictated by the specific research objective. A primary consideration is whether the experimental goal requires clean, comparable readouts for metabolic processes, such as lipid handling and insulin signalling. This contrasts with objectives requiring the inclusion of stromal crosstalk, capillary networks, and innate immune components within the microtissue. The choice of SVF or ADSC fundamentally determines the subsequent experimental outcomes. Definitions, isolation methods, and comparative benefits and drawbacks of each source must be weighed. Specific model examples demonstrate these different applications in practice.
SVF represents the heterogeneous single-cell pellet released from adipose tissue after enzymatic collagenase digestion. It is not a single cell type but rather a complex population. This mixture includes endothelial cells, pericytes, adipocyte progenitors, fibroblasts, mast cells, and macrophages. For flow cytometry, the stromal progenitor gate is typically enriched by excluding hematopoietic and endothelial markers (CD45⁻, CD235a⁻, CD31⁻) and selecting CD34⁺ cells. The isolation protocol involves mincing lipoaspirate or surgical fat. This is followed by collagenase digestion, neutralization, and centrifugation. Mature adipocytes become buoyant, while the cell pellet at the bottom constitutes the SVF.
ADSCs are functionally distinct from SVF. They are defined as the plastic-adherent, culture-expanded stromal cell population that is derived from SVF. The methodology involves plating the fresh SVF and then removing non-adherent cells by rinsing. The remaining adherent, spindle-shaped population is subsequently expanded. After several passages, a reproducible, MSC-like immunophenotype emerges. This is characterized by positivity for CD13, CD29, CD44, CD73, CD90, and CD105, while remaining negative for hematopoietic and endothelial markers. A useful distinction is CD34 positivity at early passages, which wanes with time in culture. This population serves as a consistent, bankable cell source for engineered 3D systems.
The heterogeneous composition of SVF allows for self-organization within 3D constructs. This mixture enables the formation of endothelial strands, which are subsequently wrapped by perivascular cells. Resident immune cells also contribute to the cytokine milieu. Adipocyte progenitors then differentiate within this shared microenvironment. If the research objective involves vascular remodelling, chronic low-grade inflammation, oxygen tension, or emergent multicellular behaviours, SVF provides a more comprehensive starting population. SVF is well-suited for organoid models that are designed to preserve this heterogeneity. This approach is advantageous for complex disease modelling where the endogenous niche is required to contribute to the phenotype.
ADSCs are preferable when study parameters prioritize standardization, repeatability, and scalability. This population can be readily expanded, cryopreserved, and applied in multi-donor panels. They can be used to generate spheroids or hydrogel constructs with high uniformity. If the primary endpoints are quantitative metabolic metrics, ADSCs represent the more practical selection. This allows for achieving comparable data across numerous conditions, such as measuring lipid accumulation, adipokine secretion, lipolysis, or insulin signalling. They also integrate predictably into microphysiological systems and decellularized adipose hydrogels, making them suitable for screening workflows.
The defining strength of SVF, its native heterogeneity, also presents challenges. Donor variability is often wider in SVF preparations compared to cultured ADSCs. This can complicate comparisons between experiments. The presence of immune components within the mixed population complicates allogeneic applications, where cells from one donor are used in a different recipient system. Furthermore, the enzymatic isolation protocol itself may be subject to stricter regulatory categories. This is a consideration for clinical translation unless closed devices or alternative mechanical methods are used. These alternative mechanical methods, however, may sacrifice the total cell yield obtained from the tissue.
The primary advantages of ADSC are standardization and logistical ease, as they demonstrate reliable expansion and are amenable to cryobanking. However, limitations relate to biological fidelity. Extended time in culture can induce phenotypic shifts in the cells. Monocultures of ADSCs inherently under-represent the complex tissue microenvironment. They specifically lack the immune and endothelial compartments. These populations must be exogenously added back into the culture if their interactions are being studied. A major difficulty for both SVF and ADSC sources is that achieving mature, long-lived unilocular adipocytes (fat cells with a single large lipid droplet) in vitro remains a considerable challenge.
For studies centred on quantitative metabolic readouts, ADSCs are the logical first choice. This is especially true in screening workflows. These readouts include triglyceride handling, adiponectin or leptin output, lipolysis (FFA flux), and insulin-pathway responsiveness. ADSCs form uniform spheroids or organoids and populate hydrogels consistently. This provides stable endocrine and metabolic data across time and different donors. The uniformity allows the mapping of dose-response curves for compounds, such as PPARγ agonists. It also supports tracking insulin-stimulated endpoints at higher throughput. Several studies provide frameworks for these ADSC-based models, often using scaffold-free pipelines like hanging drops or agar support.
If the biological signal being studied is dependent on intercellular crosstalk, SVF is the appropriate starting material. This applies to interactions involving capillaries, perivascular cells, or resident immune cells. The organoids that result from SVF preserve these cellular interactions. This is the preferred route for models of inflammation-modulated insulin resistance. It is also used for studies of angiogenesis and barrier function, or investigations into how oxygen supply dictates lipid storage and survival under stress. Perfusion systems are a natural application for these complex models. Some studies show SVF organoids develop visible CD31⁺ vascular strands and αSMA⁺ perivascular coverage that persist through adipogenesis.
Yes, SVF and ADSC should not be viewed as rival methodologies. They are distinct tools for different experimental objectives. One pattern gaining traction is a two-step approach: discovery with SVF, followed by measurement with ADSC. An SVF organoid is first employed to capture a complex phenomenon and identify the required cellular players. Once the critical mechanisms are known, a minimal system can be reconstructed. This new system uses ADSCs plus the specific endothelial or immune partners identified earlier. This allows for a more controlled, higher-throughput screen. Many research programmes use both: SVF for discovery of complex phenomena, and ADSC to measure the mechanisms with high control.