HER2-positive breast cancer represents about one quarter of breast malignancies and is driven by amplification of the ERBB2 gene encoding the HER2 receptor tyrosine kinase. Despite the success of targeted agents such as trastuzumab, pertuzumab, and antibody–drug conjugates, resistance and heterogeneous therapeutic response remain major concerns. Conventional two-dimensional cultures and xenografts have provided valuable molecular data but cannot reproduce the structural and signaling complexity of human tumors. The development of HER2+ organoids, including cell-line derived organoids, patient-derived organoids, and breast cancer-on-chip systems, has opened more physiologically relevant avenues for research. These breast cancer organoid models maintain native tissue polarity, extracellular matrix interactions, and intratumoral heterogeneity, allowing analysis of HER2 signaling dynamics and drug penetration within realistic gradients. In parallel, microfluidic breast cancer-on-chip approaches integrate vascular and immune elements under controlled perfusion, supporting studies on antibody delivery, immune engagement, and adaptive resistance.
Together, these new methodologies are reshaping preclinical HER2 research. By combining genetic fidelity with microenvironmental control, HER2+ organoids provide an experimental framework that bridges mechanistic studies with translational drug discovery and contributes to the refinement of personalized therapeutic strategies.
Learn more about our ready to use breast cancer organoid models.
Cell-line derived organoids in HER2-positive breast cancer
Cell-line derived organoids, reconstructed from established HER2-overexpressing cell lines, have become essential for studying receptor signaling, tumor architecture, and therapeutic response in controlled in vitro systems. These models reproduce aspects of tumor morphology and microenvironment organization that are absent in monolayer cultures, allowing us to investigate how HER2 signaling operates within spatial constraints and varying nutrient or oxygen gradients.
Architecture-dependent signaling and heterogeneity
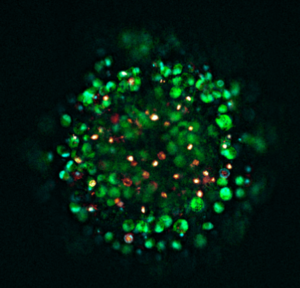
Recent analyses (pre-print) using BT474 and SKBR3 cell-line derived organoids have revealed major differences in tissue organization and receptor signaling despite both being classified as HER2-positive. BT474 cells tend to form compact, spherical aggregates with defined polarity and reduced AKT phosphorylation, while SKBR3 cultures develop loosely organized structures with enhanced HER2 expression but diminished cohesion. Confocal microscopy has shown that HER2 remains concentrated at the cell membrane across both models, although receptor density decreases toward the center of larger aggregates. These differences in morphology and signal intensity demonstrate how structural organization modulates HER2 activity, supporting the view that tumor geometry and cell–cell contact influence oncogenic signaling and drug response(1).
Drug penetration and therapeutic efficacy
Drug screening studies have highlighted that antibody-based therapies behave differently in 3D architecture. The use of a HER2-directed immunotoxin (4D5scFv-PE40) demonstrated that its cytotoxic effect was several orders of magnitude lower in HER2+ cell-line derived organoids than in equivalent 2D cultures. Limited diffusion restricted the compound to the outer layers of the organoid, leaving central cells largely unaffected. Similar findings were reported for trastuzumab, where microfluidic devices generating linear drug gradients revealed substantial differences in concentration-dependent responses. These observations indicate that reduced drug penetration may contribute to resistance observed in clinical settings, particularly in dense tumor regions with poor vascularization(2).
Multi-cell type spheroids and tumor microenvironment
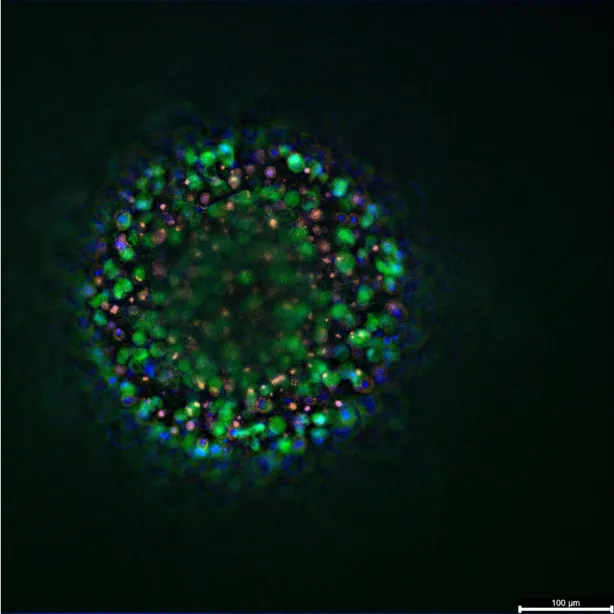
Incorporation of stromal and immune components has further expanded the predictive power of cell-line derived organoids. A recent multicellular “tetraculture” model combined HER2-positive epithelial cells with fibroblasts, macrophages, and endothelial cells. The resulting structures recapitulated patient-specific tissue organization, where fibroblasts and macrophages localized in distinct zones depending on tumor subtype. In HER2-positive BT474 organoids, cancer-associated fibroblasts aggregated centrally, creating compact structures with hypoxic cores. In contrast, SKBR3 organoids showed a more dispersed stromal pattern and peripheral macrophage accumulation. These arrangements produced spatial gradients in viability and metabolic activity, resembling those observed in tumor biopsies(3).
Such multicellular organoids are now being used to evaluate HER2-targeted agents and immune-modulating treatments in environments closer to the native tumor niche. They allow investigation of how extracellular matrix composition, fibroblast signaling, and immune infiltration alter drug sensitivity. By maintaining a reproducible structure derived from standardized cell lines, they also serve as accessible preclinical tools for comparing treatment regimens before advancing to patient-derived systems.
Overall, cell-line derived organoids provide an adaptable, reproducible foundation for mechanistic studies of HER2-driven breast cancer. They capture essential features of receptor regulation and therapeutic response, bridging the simplicity of monolayer cultures with the complexity of patient-derived organoids, and supporting the refinement of HER2+ organoid-based drug discovery pipelines.
Patient-Derived Organoids (PDOs) in HER2-Positive Breast Cancer
Patient-derived organoids (PDOs) established from HER2-positive breast tumors have become a central component of translational oncology. Derived directly from patient tissue or metastatic biopsies, these models preserve the histological and molecular identity of the tumor, including ERBB2 amplification, hormone receptor status, and genomic alterations. They provide an opportunity to observe patient-specific tumor behavior under controlled experimental conditions while maintaining the genetic diversity that defines clinical heterogeneity.
Capturing tumor heterogeneity and receptor status
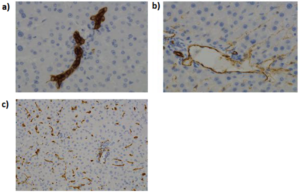
Early collections of breast cancer organoid biobanks demonstrated that organoids generated from diverse subtypes, including HER2-enriched tumors, retained the architecture, receptor expression, and growth characteristics of their tissue of origin. Importantly, these cultures maintain genomic stability across passages, confirming their value for longitudinal analysis of drug sensitivity and resistance. HER2+ PDOs have been shown to reproduce not only receptor overexpression but also the variable responses observed among patients treated with HER2-directed therapies(4).
Drug screening and precision medicine
PDOs allow direct assessment of therapeutic sensitivity on patient-derived tissues, offering a route to individualized treatment design. A 2022 study using breast cancer xenografts and organoids demonstrated real-time drug testing that guided successful therapy in a metastatic case, achieving a complete clinical response. The same concept now informs HER2+ organoid research, where patient-derived cultures are screened with antibodies, kinase inhibitors, and conjugates to identify optimal regimens. Notably, organoids from tumors that lost HER2 after therapy required neuregulin-1 for growth, revealing adaptive signaling changes and providing a model to evaluate HER2-independent treatment strategies(5).
Therapeutic response and resistance mechanisms
Beyond single-agent testing, PDOs enable combination therapy evaluation under conditions that approximate in vivo heterogeneity. Studies using organoids from HER2-low and HER2-amplified tumors have compared the effects of trastuzumab, tyrosine kinase inhibitors, and antibody–drug conjugates. While trastuzumab alone often produced limited cytotoxicity in organoids with low receptor expression, the addition of pan-HER inhibitors such as neratinib enhanced growth inhibition and delayed recovery, demonstrating the capacity of PDOs to reveal synergistic effects and resistance mechanisms within defined molecular contexts(6).
Immunotherapy testing in organoids
These organoid systems also support the exploration of immunotherapies. Recent experiments employing HER2-expressing organoids co-cultured with chimeric antigen receptor (CAR) natural killer (NK) or T cells have provided a human-relevant setting to study immune infiltration and cytotoxic activity. Within these 3D cultures, engineered immune cells successfully penetrated the organoid matrix and selectively eliminated HER2-positive cells, demonstrating measurable antitumor efficacy and cytokine release patterns like those observed in preclinical animal models. This type of approach bridges immune-oncology and patient-specific modeling, allowing assessment of immunotherapy potency and potential safety concerns in a contained, reproducible system(7).
Collectively, HER2+ PDOs represent a new methodology that aligns clinical observations with experimental data. They retain tumor-specific genotypes and phenotypes, allow evaluation of drug combinations under realistic diffusion and signaling conditions, and reveal mechanisms of acquired resistance that are otherwise difficult to capture. As biobanks expand and culture protocols standardize, patient-derived organoids are expected to play a central role in precision oncology, supporting individualized treatment decisions and the discovery of new therapeutic strategies for HER2-positive breast cancer.
Breast Cancer-on-Chip Systems
Microfluidic breast cancer-on-chip models integrate 3D cell culture, controlled perfusion, and multicellular architecture to recreate tumor behavior under dynamic conditions. When applied to HER2+ organoids, these systems offer a means to study vascularization, immune infiltration, and drug distribution with spatial and temporal precision. By combining HER2-overexpressing tumor cells, stromal elements, and microvascular endothelium within perfusable channels, researchers can reproduce gradients of oxygen, nutrients, and therapeutics that are otherwise absent in static organoid culture.
Vascular perfusion and drug delivery
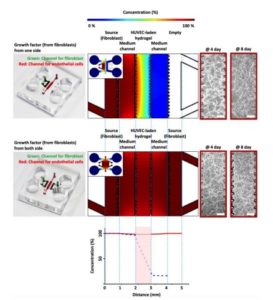
A major advantage of microfluidic breast cancer-on-chip systems is their capacity to reproduce vascular perfusion, providing a dynamic environment that reflects drug transport and clearance in vivo. Nashimoto et al. developed a chip where breast cancer–fibroblast organoids were enclosed by self-assembled human endothelial networks, allowing delivery of chemotherapeutic agents through perfused microvessels. Under flow, cytotoxic responses were markedly weaker and more gradual than in static cultures, suggesting reduced overall drug accumulation within tumor tissue(8). Similarly, Pradhan et al. reported diminished cancer cell death under continuous perfusion, despite equivalent drug concentrations(9). These studies demonstrate how fluid dynamics influence therapeutic exposure, revealing that conventional static assays may overestimate efficacy. By incorporating endothelial barriers and nutrient gradients, perfused HER2+ organoids develop necrotic cores and pharmacokinetic profiles closer to patient tumors, providing a refined model for evaluating HER2-targeted agents and optimizing delivery strategies in complex tissue environments.
Modeling tumor-stroma and invasion
Modeling Tumor–Stroma and Invasion: Microfluidic chips provide fine spatial control to study interactions between tumor cells and stromal components. For luminal/HER2+ breast cancer, Gioiella et al. designed a chip with two adjacent chambers separated by microscale pillars: one side was seeded with MCF-7 cancer cells (ER+/HER2-low luminal line) and the other with normal fibroblasts. The porous divider permitted cell–cell contact and molecular crosstalk. Using this setup, they showed that co-culture with tumor cells induced fibroblast activation to a pro-tumorigenic myofibroblast state (evidenced by upregulation of αSMA and growth factors). This mimics the desmoplastic reaction seen in actual tumors(10). Other chip systems have been used to examine cancer cell migration and invasion in real time. In one design, a central 3D region held a spheroid of tumor cells (either HER2+ MCF-7 or triple-negative MDA-MB-231) embedded in matrix, while adjacent channels contained collagen or stromal cells; investigators could monitor tumor cells invading out of the spheroid into the matrix and quantify how different conditions (e.g. presence of fibroblasts or gradient of attractants) affected invasion. These organ-on-chip approaches provide insights into the invasive behavior of HER2+ tumors and how the microenvironment (such as cancer-associated fibroblasts) may facilitate or restrain local invasion.
Immune interactions and therapy on chip
Integration of immune components adds an additional layer of relevance. Platforms combining vascularized HER2+ tumor organoids with a perfusable endothelial interface have enabled real-time tracking of immune cell extravasation. Perfused T or NK cells, including those engineered with chimeric antigen receptors (CARs), can be observed as they traverse the endothelial layer and infiltrate the tumor compartment. This setup has been used to evaluate the efficiency and selectivity of HER2-directed CAR-T and CAR-NK cells in a physiologically relevant context. The approach also permits analysis of cytokine release, immune activation, and potential off-target interactions, offering a human-specific platform for refining cellular immunotherapies before clinical translation(11).
Metastasis and crosstalk
Some new methodologies extend beyond primary tumor modeling to investigate metastasis and organ-specific colonization. Multi-organ chips may connect HER2+ breast cancer organoids with compartments mimicking secondary sites such as bone or brain tissue, enabling analysis of metastatic seeding, dormancy, and therapeutic intervention across connected microenvironments. By linking tumor growth with systemic responses under perfusion, these devices represent an intermediate level between organoid culture and animal studies.
Together, breast cancer-on-chip technologies complement cell-line and patient-derived organoids by introducing dynamic control and physiological fluid exchange. They support quantitative analysis of drug transport, immune engagement, and microenvironmental adaptation under human-relevant conditions. As standardization advances, these microengineered models are expected to become integral components of preclinical pipelines, refining predictions of therapeutic performance and resistance in HER2-positive breast cancer.
Conclusion
The emergence of HER2+ organoids has reshaped preclinical breast cancer research by offering physiologically relevant systems that bridge molecular studies and clinical application. Through cell-line derived organoids, researchers can analyze receptor signaling, architectural organization, and drug penetration in a controlled yet structured environment. Patient-derived organoids extend these observations to the clinical level, preserving tumor heterogeneity, mutational profiles, and therapy-induced adaptations, thereby providing an essential link to personalized medicine.
In parallel, breast cancer-on-chip technologies add microenvironmental complexity through vascular flow, stromal components, and immune interactions. These microengineered systems reproduce the spatial and temporal dimensions of tumor progression, capturing dynamic processes such as drug delivery, immune infiltration, and metastatic spread. Together, these new methodologies provide a coherent experimental framework for understanding HER2-driven biology and refining targeted therapy development.
The convergence of organoid culture and microfluidic engineering is expected to redefine how therapeutic efficacy and resistance are evaluated before clinical testing. As culture protocols and bioengineering platforms become standardized, integration of omics data, patient-derived cells, and automated image-based analytics will further strengthen their predictive power. HER2+ organoids thus represent a central step toward a more accurate, human-based preclinical model system that can accelerate drug discovery and improve treatment outcomes in HER2-positive breast cancer.
References
- Arnaldi P, Zotti VD, Bellese G, Gagliani MC, Orecchia P, Castagnola P, et al. 3D Breast Cancer Spheroids Reveal Architecture-Dependent HER2 Expression and Signaling [Internet]. Biology and Life Sciences; 2025 [cité 22 oct 2025]. Disponible sur: https://www.preprints.org/manuscript/202510.0712/v1
- Balalaeva IV, Sokolova EA, Puzhikhina AD, Brilkina AA, Deyev SM. Spheroids of HER2-Positive Breast Adenocarcinoma for Studying Anticancer Immunotoxins In Vitro.
- Piwocka O, Sterzyńska K, Malińska A, Suchorska WM, Kulcenty K. Development of tetraculture spheroids as a versatile 3D model for personalized breast cancer research. Sci Rep. 28 juill 2025;15(1):27449.
- Sachs N, De Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. janv 2018;172(1‑2):373-386.e10.
- Guillen KP, Fujita M, Butterfield AJ, Scherer SD, Bailey MH, Chu Z, et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer. 24 févr 2022;3(2):232‑50.
- Arshad M, Azad A, Chan PYK, Vigneswara V, Feldinger K, Nafi SNM, et al. Neratinib could be effective as monotherapy or in combination with trastuzumab in HER2-low breast cancer cells and organoid models. Br J Cancer. 29 juin 2024;130(12):1990‑2002.
- Röder J, Alekseeva T, Kiefer A, Kühnel I, Prüfer M, Zhang C, et al. ErbB2/HER2-targeted CAR-NK cells eliminate breast cancer cells in an organoid model that recapitulates tumor progression. Mol Ther. août 2025;33(8):3559‑75.
- Nashimoto Y, Okada R, Hanada S, Arima Y, Nishiyama K, Miura T, et al. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials. janv 2020;229:119547.
- Pradhan S, Smith AM, Garson CJ, Hassani I, Seeto WJ, Pant K, et al. A Microvascularized Tumor-mimetic Platform for Assessing Anti-cancer Drug Efficacy. Sci Rep. 16 févr 2018;8(1):3171.
- Gioiella F, Urciuolo F, Imparato G, Brancato V, Netti PA. An Engineered Breast Cancer Model on a Chip to Replicate ECM‐Activation In Vitro during Tumor Progression. Adv Healthc Mater. déc 2016;5(23):3074‑84.
- Maulana TI, Teufel C, Cipriano M, Roosz J, Lazarevski L, Van Den Hil FE, et al. Breast cancer-on-chip for patient-specific efficacy and safety testing of CAR-T cells. Cell Stem Cell. juill 2024;31(7):989-1002.e9.
FAQ
HER2-positive breast cancer accounts for approximately one-quarter of breast malignancies. This cancer type is driven by the amplification of the ERBB2 gene. Although targeted agents are used, resistance to treatment and varied therapeutic responses remain serious problems. Traditional research methods, such as two-dimensional cultures and xenografts, have provided molecular information. These older models cannot, however, reproduce the intricate structural and signaling organization found in human tumours. New experimental systems were developed to better represent human tumour physiology. These systems include organoids and breast cancer-on-chip devices. They are intended to provide more applicable avenues of research into HER2 signaling and drug responses.
HER2-positive organoids provide experimental systems that are considered more physiologically relevant than conventional cultures. These models, which include cell-line derived and patient-derived organoids, maintain features of the original tissue. These features include native polarity, interactions with the extracellular matrix, and intratumoral heterogeneity. This structure permits analysis of HER2 signaling dynamics. It also allows study of drug penetration within realistic gradients. Microfluidic breast cancer-on-chip approaches are used to integrate vascular and immune components under controlled flow. These systems support studies on antibody delivery, immune cell engagement, and adaptive resistance. By combining genetic fidelity with microenvironmental control, these methodologies are altering preclinical HER2 research.
Cell-line derived organoids are used to study how tumour architecture affects signaling. Recent analyses compared BT474 and SKBR3 organoids, as both are HER2-positive lines. Large differences in tissue organization and receptor signaling were found. BT474 cells were observed to form compact, spherical aggregates. These structures had defined polarity and reduced AKT phosphorylation. In contrast, SKBR3 cultures developed loosely organized structures. These showed increased HER2 expression but diminished cohesion. Confocal microscopy revealed that HER2 receptor density decreases toward the center of larger aggregates in both models. These morphological differences show how structural organization modulates HER2 activity. Tumour geometry and cell-to-cell contact appear to affect oncogenic signaling.
Drug screening studies show that antibody-based therapies behave differently in a three-dimensional architecture. The cytotoxic effect of a HER2-directed immunotoxin was tested. Its effect was found to be several orders of magnitude lower in HER2-positive cell-line derived organoids compared to 2D cultures. This difference was attributed to limited diffusion. The compound was restricted to the outer layers of the organoid, so central cells were largely unaffected. Similar findings were reported for trastuzumab. Microfluidic devices that generate linear drug gradients showed large differences in concentration-dependent responses. These observations suggest that reduced drug penetration may be a reason for resistance seen in clinical settings. This is particularly true in dense tumour regions that have poor vascularization.
A “tetraculture” model is a type of multicellular organoid. It is designed to incorporate stromal and immune components, which expands the predictive power of cell-line derived organoids. This model combines HER2-positive epithelial cells with fibroblasts, macrophages, and endothelial cells. The resulting structures were found to recapitulate patient-specific tissue organization. The localization of fibroblasts and macrophages differed depending on the tumour subtype. As an illustration, in HER2-positive BT474 organoids, cancer-associated fibroblasts aggregated in the center. This created compact structures that contained hypoxic cores. In contrast, SKBR3 organoids showed a more dispersed stromal pattern and peripheral macrophage accumulation. These different arrangements produced spatial gradients in viability and metabolic activity.
Patient-derived organoids (PDOs) are established directly from patient tissue, including metastatic biopsies. A main feature of these models is that they preserve the histological and molecular identity of the original tumour. This includes ERBB2 amplification, hormone receptor status, and other genomic alterations. PDOs provide an opportunity to observe patient-specific tumour behaviour under controlled experimental conditions. They also maintain the genetic diversity that defines clinical heterogeneity. Organoid biobanks have shown that these cultures retain the architecture, receptor expression, and growth characteristics of their tissue of origin. In addition, PDOs have been shown to maintain genomic stability across passages, which makes them suited for longitudinal analysis.
Patient-derived organoids are used for direct assessment of therapeutic sensitivity on tissues from a patient. This provides a route to individualized treatment design. One 2022 study used organoids to guide therapy in a metastatic case, achieving a complete clinical response. These models are also used to study resistance. Organoids grown from tumours that had lost HER2 after therapy were analyzed. These cultures required neuregulin-1 for growth, which revealed adaptive signaling changes. This provided a model to evaluate HER2-independent treatment strategies. PDOs also permit combination therapy evaluation. Studies using organoids from HER2-low and HER2-amplified tumours compared different drugs. Although trastuzumab alone often had limited cytotoxicity, adding pan-HER inhibitors like neratinib increased growth inhibition and delayed recovery.
A main advantage of microfluidic breast cancer-on-chip systems is the ability to reproduce vascular perfusion. This creates a situation that reflects drug transport and clearance. One study developed a chip where breast cancer–fibroblast organoids were enclosed by self-assembled human endothelial networks. Chemotherapeutic agents were delivered through these perfused microvessels. Under flow conditions, cytotoxic responses were observed to be markedly weaker and more gradual than in static cultures. This finding suggests reduced overall drug accumulation within the tumour tissue. Another study reported diminished cancer cell death under continuous perfusion, even with equivalent drug concentrations. These studies show how fluid dynamics affect therapeutic exposure. Conventional static assays may overestimate efficacy.
Microfluidic chips provide fine spatial control, which is used to study interactions between tumour cells and stromal components. One design used a chip with two adjacent chambers separated by microscale pillars. One side was seeded with MCF-7 cancer cells and the other with normal fibroblasts. This porous divider permitted cell-to-cell contact and molecular crosstalk. It was shown that co-culture with tumour cells induced fibroblast activation to a pro-tumorigenic myofibroblast state. This process resembles the desmoplastic reaction seen in actual tumours. Other chip systems are used to examine cancer cell migration and invasion in real time. A central region holds a spheroid of tumour cells embedded in matrix, while adjacent channels contain collagen or stromal cells. Investigators can monitor tumour cells invading out of the spheroid and quantify how different conditions affect this invasion.
Yes, both patient-derived organoids and chip systems are used for immunotherapy research. HER2-expressing PDOs have been co-cultured with chimeric antigen receptor (CAR) natural killer (NK) or T cells. In these experiments, the engineered immune cells successfully penetrated the organoid matrix. They also selectively eliminated HER2-positive cells. This method shows measurable antitumor efficacy and cytokine release patterns. Breast cancer-on-chip platforms are also used. Systems combining vascularized HER2-positive tumour organoids with a perfusable endothelial interface have been developed. This setup allows for real-time tracking of immune cell extravasation as cells traverse the endothelial layer. It is used to evaluate the effectiveness and selectivity of HER2-directed CAR-T and CAR-NK cells in a human-specific context.