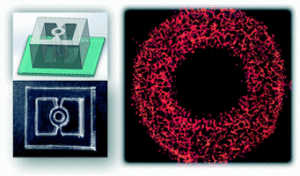
As the global obesity crisis intensifies, the functional aspect of brown adipose vs white adipose tissue has become a pivotal focus for metabolic‑disease drug discovery. Brown adipose tissue (BAT) liberates calories as heat, whereas white adipose tissue (WAT) stores excess energy and, when dysregulated, drives insulin resistance, non‑alcoholic fatty‑liver disease and cardiometabolic complications. Understanding this adipose tissue difference therefore represents a major opportunity for pharma, biotech and CROs.
Understanding Adipose Tissue: An Overview
Adipose tissue is now recognized as a highly dynamic endocrine organ, not a passive lipid store. It is a mosaic of mature adipocytes interwoven with immune, endothelial and stromal-vascular cells, all of which coordinate whole-body energy and nutrient handling. White adipose tissue (WAT) comprises large unilocular adipocytes that esterify incoming fatty acids and glucose into triglycerides, but it also secretes more than 50 bioactive molecules (including leptin, adiponectin and resistin) that fine-tune appetite, insulin action and inflammation. Brown adipose tissue (BAT), by contrast, is densely vascularized, richly innervated and packed with mitochondria containing uncoupling protein-1 (UCP1); this machinery allows rapid substrate oxidation and non-shivering thermogenesis that dissipates excess calories as heat.
Both depots are remarkably plastic. Cold exposure, adrenergic tone or certain hormones can induce “beige” adipocytes within WAT, whereas chronic over-nutrition drives WAT hypertrophy and immune infiltration. Conversely, BAT volume and activity decline with age and thermoneutral living. Beyond lipid balance, adipose tissue contributes directly to glucose disposal via GLUT4 and even serves as a glucose sink when sympathetic activity is high. This interplay of cellular heterogeneity, secretory output and environmental responsiveness underlies why manipulating adipose biology has become central to modern metabolic-disease therapeutics.
Emerging human-based systems are beginning to bridge this gap. Organoids, microphysiological “organ-on-chip” platforms, and computational NAMs can capture human-specific biology earlier in the pipeline, potentially flagging failures before expensive animal studies or Phase I trials. Recognizing this, the FDA has issued a 2025 roadmap that outlines a stepwise reduction (but not elimination) of mandatory animal tests, contingent on the validation of such methodologies (3). In this evolving landscape, adipose-tissue organoids occupy a sweet spot: they retain the multicellular complexity needed to model chronic metabolic disease yet can be produced in sufficient quantities for medium-throughput screening. As the field converges on standardized culture protocols and quality-control metrics, these models are poised to make preclinical evaluation both more predictive and more humane.
Learn more about our ready to use Adipose organoid plate.
The Role of Brown Adipose Tissue in Metabolism
Brown adipose tissue (BAT) is a metabolically voracious “glucose- and lipid-sink” that defends both body temperature and cardiometabolic health. Cold or β-adrenergic stimulation activates the canonical β3-AR → cAMP → PKA cascade, releasing fatty acids that directly engage uncoupling-protein-1 and dissipate the mitochondrial proton gradient as heat. Because UCP1-driven respiration can consume circulating glucose and free fatty acids at gram-scale rates, active BAT clears up to 75 % of a glucose load and nearly half of post-prandial triglycerides in mouse models, thereby lowering glycaemia and plasma lipids. Beyond combustion, BAT is an endocrine organ: batokines such as FGF21, IL-6, NRG4 and the lipokine 12,13-diHOME signal to liver, heart and skeletal muscle to enhance insulin sensitivity, curb hepatic lipogenesis and boost cardiac output. BAT transplants or human pluripotent-stem-cell-derived brown adipocytes restore euglycemia and blunt atherosclerosis in rodent models, underscoring its therapeutic potential(1). Collectively, BAT couples rapid substrate oxidation with hormone-like communication to orchestrate whole-body energy balance.
The Function of White Adipose Tissue in Energy Storage
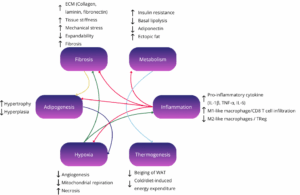
White adipose tissue (WAT) is the principal caloric reservoir, capturing excess plasma glucose and free fatty acids and re-esterifying them into triglycerides under the control of lipogenic enzymes and the sympathetic nervous system(2). During fasting or adrenergic stress, adipose-triglyceride-lipase and hormone-sensitive-lipase mobilize these stores to fuel peripheral tissues, illustrating WAT’s bidirectional energy buffering role. Yet it is widely accepted now that WAT is not just an energy reservoir, its unilocular adipocytes, stromal-vascular cells and resident immune cells secrete a rich cocktail of adipokines, including leptin, adiponectin, resistin, visfatin and hundreds of exosomal microRNAs, that modulate appetite, insulin action, vascular tone and even cognition. Obesity drives adipocyte hypertrophy, ECM stiffening and macrophage infiltration, shifting this secretome toward pro-inflammatory TNF-α, IL-6 and MCP-1 and precipitating systemic insulin resistance(3). Therapeutically, enhancing healthy WAT expansion (via selective PPARγ agonism), improving blood flow with incretin analogues and inducing beige conversion with cold, β3-agonists or FGF21 aim to restore its buffering and endocrine functions while preventing ectopic lipid spill-over.
Brown vs White Adipose Tissue: Key differences
Adipose-tissue organoids have evolved into a versatile pre-clinical platform that now underpins much of modern metabolic-disease research. By finely tuning the culture environment, investigators can reproduce every major facet of “unhealthy” fat: lipid-droplet hypertrophy, heightened basal lipolysis, a shift in the adiponectin-to-leptin ratio, insulin-signaling defects, and the low-grade inflammatory milieu typical of metabolically diseased adipose tissue(5). Adding pro-inflammatory cytokines or live macrophages deepens that phenotype, generating a chronic, depot-specific inflammation that is ideally suited for evaluating small-molecule or antibody therapies. Because white-adipose organoids (WAOs) can be formed in miniaturized arrays, entire chemical libraries are now screened in parallel; automated imaging and secretome read-outs swiftly flag compounds that normalize lipid storage, temper cytokine release, or restore glucose uptake.
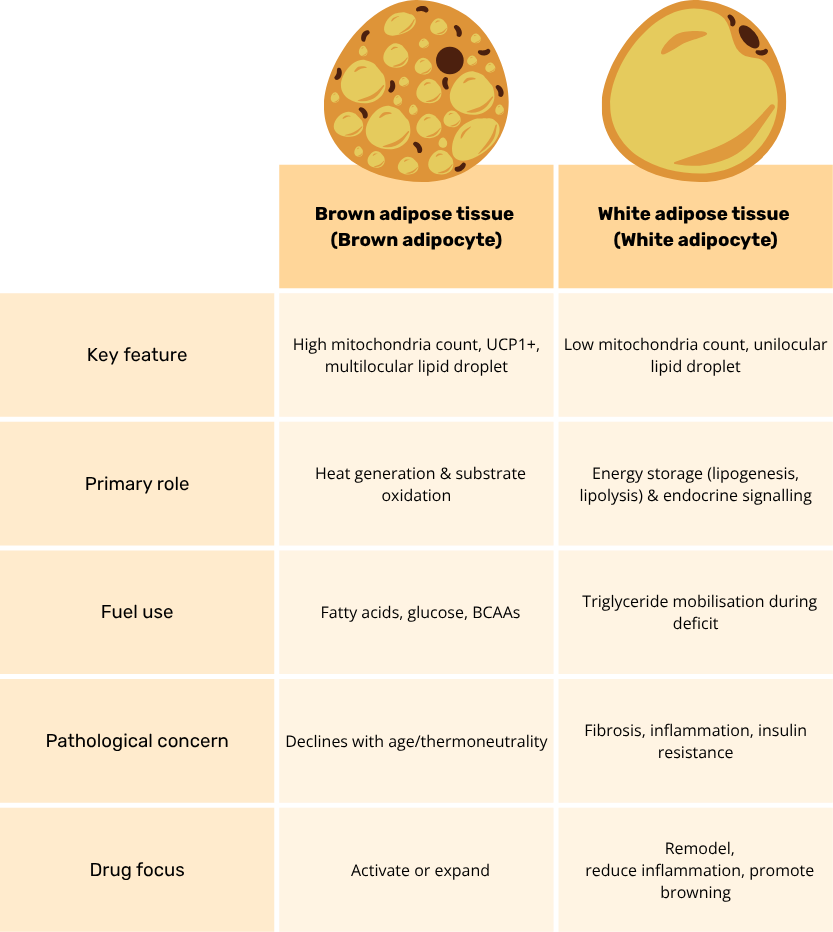
Brown vs White Adipose Tissue: Implications for Health and Disease
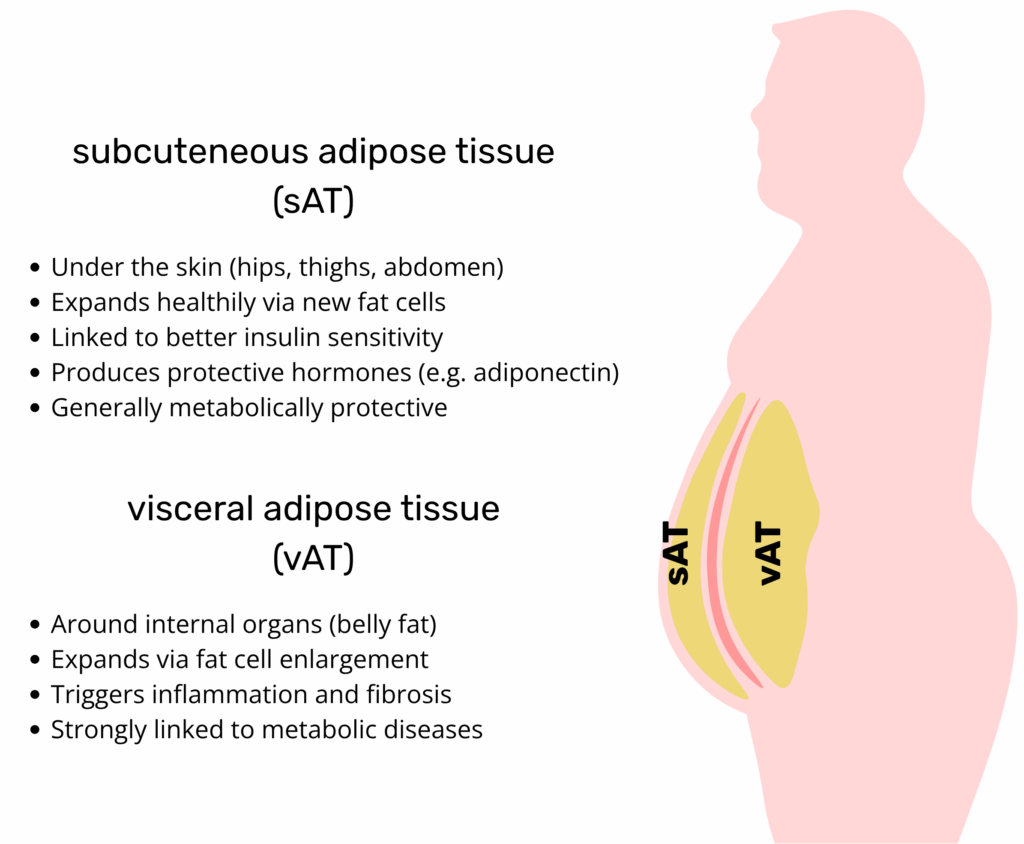
Brown vs White Adipose Tissue in terms of body distribution, and not just total fat mass, plays a central role in determining metabolic health outcomes. Visceral white adipose tissue (WAT), located deep within the abdominal cavity, is strongly associated with insulin resistance, dyslipidaemia, type 2 diabetes, and cardiovascular disease. This depot is more prone to inflammation, fibrosis, and ectopic lipid overflow, all of which contribute to systemic metabolic dysfunction. In contrast, subcutaneous WAT, particularly in gluteofemoral regions, is metabolically protective when it expands through hyperplasia rather than hypertrophy. Brown adipose tissue (BAT), though smaller in mass, exerts profound systemic effects through thermogenesis and endocrine signaling. Active BAT is associated with improved insulin sensitivity, lower triglyceride levels, and better body mass index, even in overweight individuals.
Crucially, adipose tissue is not static. Under cold exposure, β-adrenergic stimulation or certain hormones, subsets of white adipocytes adopt a thermogenic programme and become beige adipocytes. Beige cells originate from the same Myf5-negative precursors as WAT but resemble BAT in their multilocular morphology and UCP1 expression; when the stimulus is removed, they can revert to a white phenotype, highlighting the reversible nature of “browning”.
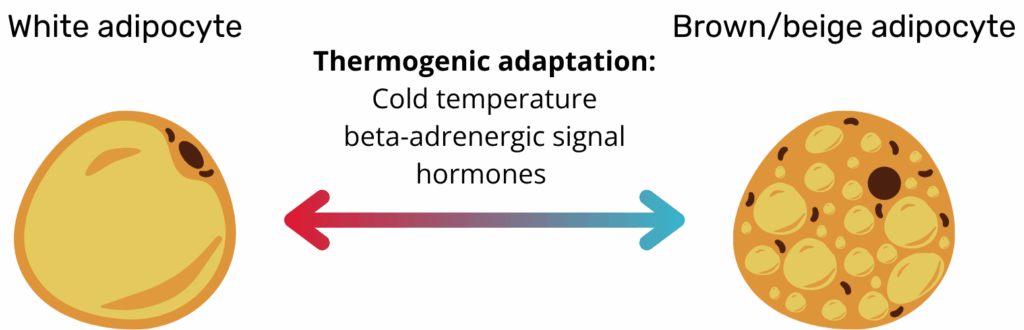
Importantly, metabolic disease risk often reflects adipose tissue function and plasticity more than quantity. Individuals with obesity who retain metabolically flexible adipose tissue, capable of expanding without triggering inflammation, can remain insulin sensitive, a phenotype termed “metabolically healthy obesity.” Conversely, lean individuals with dysfunctional fat depots may exhibit elevated cardiometabolic risk. These insights underscore the therapeutic value of improving adipose tissue quality, by reducing inflammation, restoring vascularisation, or enhancing browning, rather than focusing solely on fat mass reduction. Understanding these nuances is key to designing precision metabolic interventions(3).
Current Research Trends in Adipose Tissue Studies
Rapid advances in molecular biology, tissue engineering, and systems biology are transforming how researchers perceived Brown vs White Adipose Tissue and their roles in metabolic disease. The focus has shifted from simple fat quantification to deep phenotyping of depot-specific function, immune interactions, and secretory profiles. This has enabled more nuanced, human-relevant insights that inform therapeutic discovery.
- Browning biology – Core regulators such as PRDM16, BMP7, and PGC1α are being mapped to understand the reversible conversion of white to beige adipocytes.
- Human-relevant models – 3D adipose organoids and organ-on-chip platforms replicate human adipose function and support high-throughput drug screening, aligned with 3Rs principles.
- Secretome mapping – Proteomic tools now catalogue depot-specific signals like FGF21, NRG4, and CXCL14 that influence systemic metabolism.
- Exosome & miRNA therapeutics – Extracellular vesicles from adipocytes deliver regulatory RNA or proteins to distant organs.
- Neuro-immune interplay – Innate lymphoid cells (ILC2s), IL-33, and γδT cells are implicated in adipose innervation and thermogenic tone.
These multidisciplinary strategies are redefining adipose tissue as a complex, targetable endocrine and immunometabolic organ(1–5).
Pharmaceutical Interests in Adipose Tissue Modulation
Pharmaceutical strategies aimed at modulating adipose tissue are increasingly focused on improving both energy storage and expenditure by targeting depot-specific functions. Rather than simply reducing fat mass, modern approaches seek to restore healthy adipose tissue architecture, enhance endocrine output, and activate thermogenesis. These efforts span small molecules, biologics, gene therapies, and cell-based interventions.
Key therapeutic avenues include:
- Thermogenic activators – Compounds such as β3-adrenergic agonists and thyroid hormone analogues stimulate brown adipose tissue (BAT) activity and energy expenditure.
- Browning agents – Peptides or small molecules (e.g., FGF21 analogues, BMP7) induce beige adipocyte formation within white adipose tissue (WAT).
- Secretome mimetics – Batokines like FGF21 and NRG4, delivered as recombinant proteins or via gene therapy, extend BAT-like metabolic benefits systemically.
- Matrix & inflammation remodelers – Targeting ECM components, fibrosis, or inflammation with PPARγ modulators and anti-fibrotic agents supports healthier WAT expansion.
Molecular targets of interest:
- Thermogenic axis – UCP1, β3-adrenergic receptor, PRDM16
- Endocrine axis – FGF21–FGFR1/β-Klotho, NRG4–ErbB4
- Storage axis – PPARγ, ECM-modifying enzymes
Together, these strategies reflect a shift toward depot-specific, function-enhancing therapies in metabolic disease.(1–3)
Challenges and Opportunities: The Future of Adipose Tissue in Pharmaceutical Development
Adipose tissue biology offers compelling opportunities for metabolic disease therapy, but significant challenges remain. Inter-individual variability in brown adipose tissue (BAT) volume and activity, transient beiging responses, and depot-specific drug distribution complicate treatment efficacy and reproducibility. Browning strategies are also limited by side effects and undesirable metabolic events that will need to be addressed to offer reliable treatment options. Additionally, the inflammatory and fibrotic remodeling of white adipose tissue (WAT) in obesity hinders its adaptive expansion and endocrine function. These hurdles demand precision tools and personalised strategies.
Encouragingly, emerging technologies, such as PET/MRI imaging, single-cell and spatial omics, and machine learning-based phenotyping, enable real-time monitoring of adipose plasticity and targeted intervention. These innovations open the door to patient stratification and depot-specific drug design.
The future of adipose tissue pharmacology lies in dual-action approaches that simultaneously activate thermogenic pathways and restore healthy WAT architecture. Combining β3-agonists, browning agents, batokine mimetics, and anti-fibrotic strategies holds promise for durable, safe metabolic correction. For pharma and biotech, mastering the cellular and endocrine complexity of adipose tissue is a requirement for next-generation treatments targeting obesity, diabetes, and cardiometabolic disease.
Resources
- An SM, Cho SH, Yoon JC. Adipose Tissue and Metabolic Health. Diabetes Metab J. 30 sept 2023;47(5):595‑611.
- Ghesmati Z, Rashid M, Fayezi S, Gieseler F, Alizadeh E, Darabi M. An update on the secretory functions of brown, white, and beige adipose tissue: Towards therapeutic applications. Rev Endocr Metab Disord. avr 2024;25(2):279‑308.
- Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. févr 2022;185(3):419‑46.
- McCarthy M, Brown T, Alarcon A, Williams C, Wu X, Abbott RD, et al. Fat-On-A-Chip Models for Research and Discovery in Obesity and Its Metabolic Comorbidities. Tissue Eng Part B Rev. 1 déc 2020;26(6):586‑95.
- Huff LK, Amurgis CM, Kokai LE, Abbott RD. Optimization and validation of a fat-on-a-chip model for non-invasive therapeutic drug discovery. Front Bioeng Biotechnol. 25 juin 2024;12:1404327.
FAQ
Adipose tissue is now understood to be an endocrine organ. It is not just a passive lipid store. It is composed of a mosaic of mature adipocytes. Immune, endothelial, and stromal-vascular cells are also interwoven in the tissue. This collection of cells coordinates the body’s energy and nutrient handling. White adipose tissue (WAT) contains large unilocular adipocytes. These cells esterify fatty acids and glucose into triglycerides. WAT also secretes many bioactive molecules. These include leptin, adiponectin, and resistin. These molecules adjust appetite, insulin action, and inflammation. Brown adipose tissue (BAT) is different. It is densely vascularized and packed with mitochondria containing UCP1. This machinery permits substrate oxidation and heat generation.
Brown adipose tissue (BAT) is a "glucose- and lipid-sink." It defends body temperature and cardiometabolic health. The canonical β3-AR → cAMP → PKA cascade is activated by cold or β-adrenergic stimulation. Fatty acids are released by this cascade. These fatty acids engage uncoupling-protein-1. The mitochondrial proton gradient is dissipated as heat. Circulating glucose and free fatty acids are consumed by UCP1\-driven respiration at gram-scale rates. Active BAT is shown to clear up to 75% of a glucose load in mouse models. Nearly half of post-prandial triglycerides are also cleared. Glycaemia and plasma lipids are lowered by this action. BAT is also an endocrine organ, secreting batokines like FGF21 and IL-6 that signal to other organs.
White adipose tissue (WAT) is the body’s main caloric reservoir. Excess plasma glucose and free fatty acids are captured by it. These are re-esterified into triglycerides. This process is controlled by lipogenic enzymes. During fasting, these stores are mobilized by adipose-triglyceride-lipase and hormone-sensitive-lipase. This action fuels peripheral tissues. WAT is not just an energy store. Its unilocular adipocytes and resident immune cells secrete adipokines. These include leptin and adiponectin. Appetite and insulin action are modulated by these molecules. When obesity occurs, adipocyte hypertrophy and macrophage infiltration are driven. This shifts the secretome toward pro-inflammatory signals like TNF-α and IL-6, which precipitates insulin resistance.
The distribution of body fat is central to metabolic health outcomes. Visceral white adipose tissue (WAT) is found deep within the abdominal cavity. It is strongly associated with insulin resistance, type 2 diabetes, and cardiovascular disease. This specific depot is more prone to inflammation and fibrosis. In comparison, subcutaneous WAT is metabolically protective. This is especially true for the gluteofemoral regions. This protective state is maintained when it expands through hyperplasia (new cell growth) rather than hypertrophy (cell enlargement). Brown adipose tissue (BAT) is smaller in mass. It is associated with improved insulin sensitivity and lower triglyceride levels.
Adipose tissue is not static. Subsets of white adipocytes can adopt a thermogenic programme. This change occurs in response to cold exposure, β-adrenergic stimulation, or certain hormones. These cells become "beige" adipocytes. Beige cells come from the same Myf5-negative precursors as white adipose tissue (WAT). They resemble brown adipose tissue (BAT) in their multilocular morphology. They also show UCP1 expression. The "browning" process is reversible. When the stimulus is removed, the beige cells can revert to a white phenotype. This highlights the reversible nature of this process.
Metabolic disease risk is often a reflection of adipose tissue function and plasticity. It is not just about the total quantity of fat. Individuals with obesity who retain metabolically flexible adipose tissue can remain insulin sensitive. This phenotype is called "metabolically healthy obesity." Their tissue is capable of expanding without triggering inflammation. Conversely, lean individuals can have dysfunctional fat depots. These individuals may exhibit elevated cardiometabolic risk. These findings underscore the therapeutic value of improving adipose tissue quality. This is different from focusing solely on fat mass reduction. This includes reducing inflammation, restoring vascularisation, or enhancing browning.
The perception of brown and white adipose tissue is being transformed by advances in molecular biology and tissue engineering. The research focus has shifted away from simple fat quantification. It is now on deep phenotyping of depot-specific function, immune interactions, and secretory profiles. One area is browning biology. Regulators such as PRDM16 and BMP7 are being mapped. This is done to understand the reversible conversion of white to beige adipocytes. Another area is secretome mapping. Proteomic tools are used to catalogue depot-specific signals like FGF21 and NRG4. Exosome and miRNA therapeutics are also studied, as vesicles from adipocytes deliver regulatory RNA. Neuro-immune interplay, involving cells like ILC2s, is also investigated.
Pharmaceutical strategies are aimed at improving energy storage and expenditure. Thermogenic activators are one avenue. These are compounds, such as β3-adrenergic agonists, that stimulate brown adipose tissue (BAT) activity and energy expenditure. Browning agents are another class of compounds. These are peptides or small molecules. FGF21 analogues and BMP7 are examples. The formation of beige adipocytes within white adipose tissue (WAT) is induced by these agents. Secretome mimetics are also being studied. Batokines like FGF21 and NRG4 are delivered as recombinant proteins. These are used to extend BAT-like metabolic benefits systemically. These approaches reflect a shift toward function-enhancing therapies.
Compelling opportunities are offered by adipose tissue biology, but challenges remain. Inter-individual variability in brown adipose tissue (BAT) volume and activity is one issue. This variability, along with transient beiging responses, complicates treatment efficacy. Browning strategies are also limited by side effects. Undesirable metabolic events are a concern and will need tobe addressed. The inflammatory and fibrotic remodeling of white adipose tissue (WAT) in obesity is another hurdle. This remodeling hinders the tissue’s adaptive expansion and endocrine function. These hurdles demand the creation of precision tools and personalised strategies.
Adipose-tissue organoids have evolved into a versatile pre-clinical platform. Every major facet of "unhealthy" fat can be reproduced by finely tuning the culture environment. This includes lipid-droplet hypertrophy, heightened basal lipolysis, and insulin-signaling defects. The low-grade inflammatory milieu typical of diseased tissue can also be reproduced. Pro-inflammatory cytokines or live macrophages can be added. This deepens the phenotype, generating depot-specific inflammation. This system is suited for evaluating small-molecule or antibody therapies. Because white-adipose organoids (WAOs) can be formed in miniaturized arrays, entire chemical libraries are now screened in parallel. Compounds that normalize lipid storage or restore glucose uptake are swiftly flagged.