Introduction
The organization and function of living muscle tissues cannot be fully recapitulated by typical two-dimensional (2D) culture methods in skeletal muscle-derived cells, reducing their use in extensive physiological research. The development of a 3D culture model gives a significant potential for imitation of the live tissues and for modeling muscular disorders. This novel in vitro model enhances our awareness of the many cell kinds present in the development and interactions of skeletal muscle and of the methods by which a diseased muscle responds to new treatments.
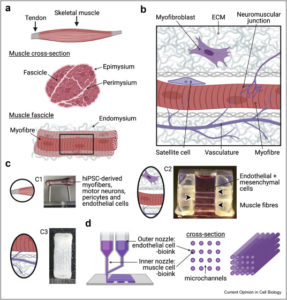
In the review, the authors begin a brief summary of the production and differentiation of skeletal myogens, followed by a discussion with human biopsy-derived myoblasts (primary or immortalized) or pluripotent stem cells on current developments. They then explore approaches to build physiologically complicated models which provide clinically meaningful phenotypic readings, which may be utilized as results measurements for the development of therapy, with their viewpoint on the future in vitro skeleton muscle models.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
Abstract
The author states that “Advanced in vitro models of human skeletal muscle tissue are increasingly needed to model complex developmental dynamics and disease mechanisms not recapitulated in animal models or in conventional monolayer cell cultures.
There has been impressive progress towards creating such models by using tissue engineering approaches to recapitulate a range of physical and biochemical components of native human skeletal muscle tissue. In this review, we discuss recent studies focused on developing complex in vitro models of human skeletal muscle beyond monolayer cell cultures, involving skeletal myogenic differentiation from human primary myoblasts or pluripotent stem cells, often in the presence of structural scaffolding support.
We conclude with our outlook on the future of advanced skeletal muscle three-dimensional cultures (e.g. organoids and biofabrication) to produce physiologically and clinically relevant platforms for disease modeling and therapy development in musculoskeletal and neuromuscular disorders.”
References
Jalal S, Dastidar S, Tedesco FS. Advanced models of human skeletal muscle differentiation, development, and disease: Three-dimensional cultures, organoids and beyond. Curr Opin Cell Biol. 2021 Aug 9;73:92-104. DOI: 10.1016/j.ceb.2021.06.004. Epub ahead of print. PMID: 34384976.
FAQ
Advanced in vitro models of human skeletal muscle tissue are increasingly required. This is because conventional monolayer cell cultures fail to reproduce complex developmental dynamics and disease mechanisms. The organisation and function of living muscle tissues cannot be fully copied by typical two-dimensional culture methods. This limitation reduces their application in extensive physiological research. Animal models also do not fully recreate these human processes. The development of 3D culture models is seen as a way to address these shortcomings. These newer models provide a better platform for the imitation of living tissues and for modelling muscular disorders.
The development of 3D culture models offers good potential for imitating live muscle tissues. These models also allow for the modelling of muscular disorders. These new in vitro systems enhance our awareness of the many cell kinds present in skeletal muscle development. They also improve the understanding of cellular interactions. An additional benefit is the ability to study the methods by which a diseased muscle responds to new treatments. Progress has been made in creating such models using tissue engineering. These approaches are used to reproduce a range of physical and biochemical components found in native human skeletal muscle.
Recent studies are focused on developing intricate in vitro models of human skeletal muscle. These models involve skeletal myogenic differentiation. This process starts from specific human cell sources. One source is human primary myoblasts. These cells may be obtained from human biopsies. Immortalized myoblasts are also used. Another source is human pluripotent stem cells. These cells are often combined with a structural scaffolding. This scaffolding provides support as the tissue develops. These current developments are aimed at building physiologically intricate models that can provide clinically applicable readings for therapy development.
The future of advanced skeletal muscle 3D cultures is explored. This outlook includes technologies such as organoids and biofabrication. The objective is to produce platforms that are physiologically and clinically applicable. These platforms are intended for disease modelling. They are also meant for therapy development, particularly for musculoskeletal and neuromuscular disorders. These approaches are designed to build intricate models that provide phenotypic readings. Such readings could be utilised as results measurements in the process of developing new treatments. This represents the next stage for in vitro skeletal muscle models.