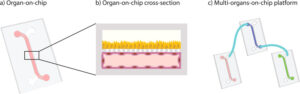
Why a skin on a chip?
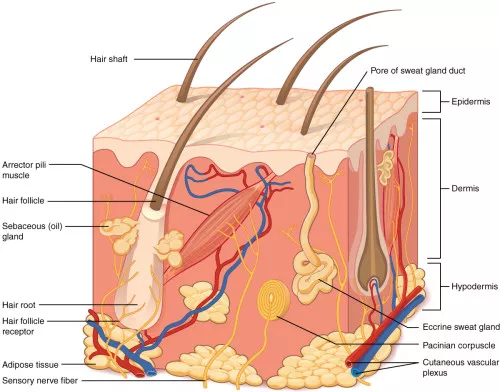
The skin is the outer envelope of the body. It contains pressure, temperature, and pain sensors and synthesizes vitamin D through sunlight. It is a complex system in terms of organization and diversity. The skin is composed of several layers (hypodermis, dermis, epidermis) and tegumentary appendages (hair, sebaceous gland). There is a temperature gradient (from 37 ° C to RT).
Current models for skin toxicity are based on animals but with 3R regulation, synthetic skins have emerged. They aim at being more predictive for skin toxicity than animal models that are fairly unpredictable due to different physiology and immunity at the level of the skin.
How to culture vascularized & immunocompetent 3D models in a standard Multiwell
What has already been achieved ?
Preamble: Because of its role, it is necessary to have an air/liquid interface and a temperature gradient for the good functionalization of the cultures and the models in vitro.
A team at Columbia University (Angela Christiano) developed a skin on a chip (in a culture insert) based on IPS cells (1). The cells are grown in 3D and have shown the ability to recreate the different layers of the skin (comparable to the human skin). Using IPS from a patient suffering from skin disease, it is now possible to test new treatments or to test molecules for cutaneous toxicity in pathological conditions (impossible using in vitro model until then).
In 2002, Schwalkwijk J established a model of psoriasis on a chip (2). The system is a classical culture of in vitro skin (primary culture of human keratinocyte), which once relocated in an inflammatory environment (cytokine IL-1, TNF-alpha, and IL-6) behaves like a “psoriasis-like” skin.
A team from the University of North Carolina (O’Neill AT) has developed a PDMS chip for the primary culture of keratinocytes in monolayers with potential application in High Throughput Screening (3).
A team from Singapore (Ramadan Q) has established skin on chip allowing the co-culture of keratinocyte (HaCaT KC) and monocyte (U937) cell lines (4). The model allows a follow-up of the transepithelial electric resistance (TEER) for the monitoring of the tight junctions and also allows the realization of an immunological test.
A Korean team (Wufuer and Lee), developed a skin on chip (5). The three layers of the skin (hypodermis, dermis, and epidermis) are reconstituted by three supports of PDMS on which the cells are cultured. Each layer is separated by a porous membrane (PET, 0.4 μM pore). This model reproduces edema and inflammation but uses cell lines (HaCaT KC, HS27 FB CL, HUVEC).
A team from Columbia University (Abaci HE) developed also one allowing the culture of full-thickness skin biopsy (6). The primary culture can be maintained for 3 weeks. It is a system without a pump allowing the study of transdermal passage/transport. Disadvantage contains only fibroblasts and keratinocytes (loss of immunity).
The same group used endothelial iPS cells in the same system. Disadvantage: a long period of culture (21d) before being able to make any experiments and a flow is applied to the culture. Advantages: the vascularization strengthens the basal lamina and allows the study of the interactions between the vascular compartment and the extracellular matrix.
A Japanese group (Takeushi S) has built a chip that mimics the vascularization of skin tissue (7). The system allows the study of the percutaneous absorption of a molecule by dosage in the vascular compartment. The culture can be maintained for 10 days, which allows the study of the interactions between the endothelial compartment and the extracellular matrix. On the other hand, it only allows a limited study of epidermal markers.
An Italian team (Imparato et al ) recreated in 2017 a fully innervated skin on chip (8). The model is composed of human dermal fibroblasts, supplemented with a keratinocyte (HaCaT) cell line for the formation of the epidermis. The neurons come from DRG rats.
TissUse has established several models of multi-organ on a chip (skin + hair follicle, skin + liver, skin + liver + kidney + intestine). The major disadvantage of these chips and the high frequency of medium change and the use of foreskin biopsy coupled with cell lines for other “organs”.
References
- PLoS ONE 8(10) (2013): e77673. Generation of 3D Skin Equivalents Fully Reconstituted from Human Induced Pluripotent Stem Cells (iPSCs)Itoh M, Umegaki-Arao N, Guo Z, Liu L, Higgins CA, Christiano AM.
- Skin Pharmacol. Appl. Skin Physiol. 15:252–61 (2002). A simple technique for high-throughput screening of drugs that modulate normal and psoriasis-like differentiation in cultured human keratinocytes. Pol A, Bergers M, van Ruissen F, Pfundt R, Schalkwijk J.
- Cytotechnology. 2008;56:197–207. O’Neill A.T., Monteiro-Riviere N.A., Walker G.M. Characterization of microfluidic human epidermal keratinocyte culture.
- Lab Chip. 2016 May 21;16(10):1899-908. doi: 10.1039/c6lc00229c. Epub 2016 Apr 21. In vitro micro-physiological immune-competent model of the human skin. Ramadan Q1, Ting FC.
- Sci. Rep. 6, 37471; doi: 10.1038/srep37471 (2016). Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Maierdanjiang Wufuer, GeonHui Lee, Woojune Hur, Byoungjun Jeon, Byung Jun Kim, Tae Hyun Choi & SangHoon Lee
- Lab Chip. 2015 Feb 7; 15(3): 882–888. Pumpless microfluidic platform for drug testing on human skin equivalents; Hasan Erbil Abaci, Karl Gledhill, Zongyou Guo, Angela M. Christiano, and Michael L. Shuler
- Biomaterials. 2017 Feb;116:48-56. doi: 10.1016/j.biomaterials.2016.11.031. Epub 2016 Nov 27. Skin integrated with perfusable vascular channels on a chip. Mori N1, Morimoto Y1, Takeuchi S
- Biomaterials. 2017 Jan;113:217-229. doi: 10.1016/j.biomaterials.2016.10.051. Epub 2016 Oct 31. In vitro activation of the neuro-transduction mechanism in sensitive organotypic human skin model. Martorina F, Casale C, Urciuolo F, Netti PA, Imparato G
FAQ
The skin is a complex system, acting as the body’s outer envelope with multiple layers, appendages, and sensors. It also has an inherent temperature gradient. "Skin on a chip" models are being developed to improve how skin toxicity is tested. Current models are often based on animals. These animal models can be unpredictable. This is because the physiology and immunity at the level of the skin are different from humans. Due to 3R regulations, synthetic skin models have emerged. The objective for these synthetic models is to be more predictive for skin toxicity than the animal models.
Specific conditions are needed for the proper functionalization of in vitro skin cultures and models. The skin’s role as an outer barrier makes these requirements important. A main requirement is the presence of an air/liquid interface. This arrangement mimics the skin’s natural exposure to the air on one side and contact with the body’s internal environment on the other. Additionally, a temperature gradient is considered necessary for good functionalization of the cultures. The skin in the body naturally has a temperature gradient, which ranges from 37°C internally down to room temperature at the surface. Replicating these two conditions is a focus for in vitro models.
Induced pluripotent stem (iPS) cells have been used to create 3D skin models. One team developed a "skin on a chip" in a culture insert using iPS cells. These cells were grown in 3D. They showed the ability to recreate the different layers of the skin in a way that was comparable to human skin. A benefit of this approach is the ability to use iPS cells derived from a patient with a skin disease. This allows for the testing of new treatments or the cutaneous toxicity of molecules in a pathological state. This was not possible with previous in vitro models. Endothelial iPS cells have also been used, which adds vascularization that strengthens the basal lamina.
Several models have been established by co-culturing different cell types. One team from Singapore created a model with both keratinocyte (HaCaT KC) and monocyte (U937) cell lines. This system permitted the monitoring of transepithelial electric resistance (TEER) and allowed immunological tests. Another Korean team reconstituted all three layers of the skin (hypodermis, dermis, and epidermis) using different cell lines (HaCaT KC, HS27 FB CL, HUVEC). These layers were cultured on three PDMS supports and separated by porous membranes. This model was able to reproduce edema and inflammation. An Italian team created a fully innervated "skin on a chip" by combining human dermal fibroblasts, a keratinocyte cell line, and neurons from DRG rats.