Introduction
The development of new techniques enabled scientists to screen more easily for C. elegans temperature sensitive mutants and by shifting temperature from permissive to sensitive background, thus precisely question the role of protein, and its absence thereof, during development, cell division, neuron formation [1].
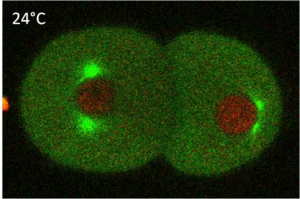
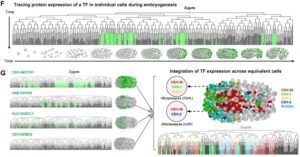
Pioneer work in C. elegans genetics and developmental biology has been initiated by Sydney Brenner since 1974. Taking advantage of the worm small number of cells, Sulston and Horvitz, back in 1977, initiated thorough cell lineage analyses. Forty years later, scientists can precisely document every single cell fate and genealogy cell tree (see figure 1) have been established.
Ultra fast temperature shift device for in vitro experiments under microscopy
C. elegans temperature sensitive mutants
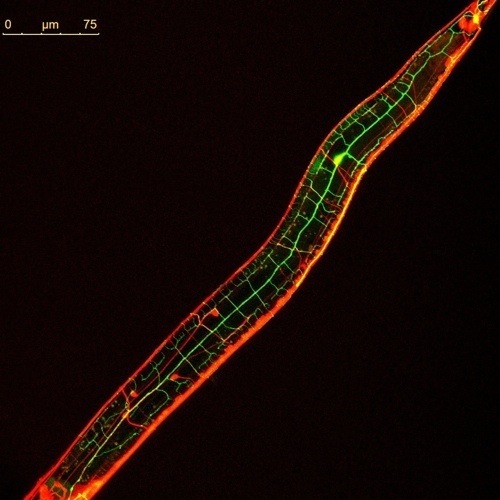
Temperature sensitive mutants represent a powerful tool to study many aspect of worm biology. Due to the technical improvements, the creation of thermo-sensitive worms has become a very easy task. For example, by combining a heat-shock protein promoter to any genes of interest allows researcher to precisely control gene activation.
The first genetic studies done in relation with the thermo sensitivity used mutants that had defects in the chemotaxis. The study focused on finding genes that are sensitive to temperatures changes [2].
As an example, a group of scientist from the team of M. Chatzigeorgiou [3] found that the exposure of C. elegans to low temperatures, from 20°C to 15°C, provokes changes in the genetic expression of trpa-1. The genetic variations lead to altered translation of the TRP-1 protein and a consequent cold-sensitive, not selective ion current.
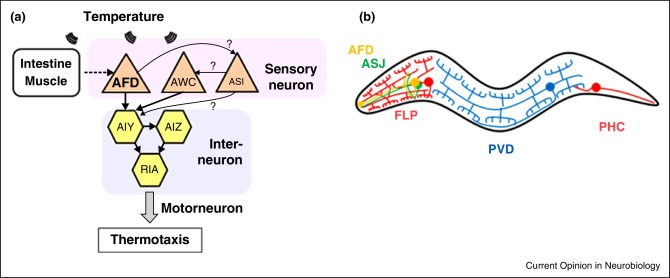
There are more methods to achieve temperature dependence in this transparent nematode. In C. elegans, the AFD neurons [4] need a cGMP-dependent network of molecules to control and regulate the ion channel. Inside this network, the temperature is the one that regulates the concentration of cGMP in the cytoplasm. The concentration level of this molecule is the one that sets how many cGMP gated ion channels are open at a certain temperature [5].
ASI chemosensory neurons have relation with thermotaxis under very specific conditions [6]. The response of ASI to temperature variations is dependent on AFD as it can be seen in the Figure 2.There are more methods to achieve temperature dependence in this transparent nematode. In C. elegans, the AFD neurons [4] need a cGMP-dependent network of molecules to control and regulate the ion channel. Inside this network, the temperature is the one that regulates the concentration of cGMP in the cytoplasm. The concentration level of this molecule is the one that sets how many cGMP gated ion channels are open at a certain temperature [5].
As examples, the Alzheimer, Parkinson, ALS and Huntington’s diseases are being studied in C. elegans [7]. Both in ALS and Huntington’s diseases, it has been shown that temperature sensitive mutant genes can act as modifiers to proteins misfolding and aggregation toxicity.
Optimizing the use of C. elegans temperature-sensitive mutants
The possibility of growing the worms at different temperatures gives the chance to control the population and to develop temperature-sensitive mutants but there are some constrains that need to take into account.
The second limitation is related with the heat shock. This technique is commonly used to analyze behaviour and response to big changes in the environment but too much time exposure to high temperatures, always more than 25°C, can increase the production of males in the population [9]. To avoid such a bias, it is important to tightly control the timing of temperature exposure.The first limitation for C. elegans populations is that if they are grown at more than 25°C the nematode can become sterile. The worms lose the capacity to reproduce; the majority of them are hermaphrodites and have the capacity to self-fertilize [8]. Hence, there is a need to get an accurate temperature control when studying worm’s physiology.
Combining fast acting embryo mutants and live-cell imaging
Given the numerous aspects of C. elegans that are controlled by temperature changes, temperature controller systems can be crutial. Until 2014, looking at early onset protein response to fast temperature change was not possible due to the lack of adequate device. A 2016 publication compared the use of two systems allowing fast temperature changes while performing high resolution live cell imaging of embryos [14].
References
- [1] O’Rourke SM et al. A survey of new temperature-sensitive, embryonic-lethal mutations in C. elegans: 24 alleles of thirteen genes. PLoS One (2011).
- [2] Dusenbery DB et al. Chemotaxis-defective mutants of the nematode Caenorhabditis elegans. Genetics (1975). Pubmed link
- [3] Chatzigeorgiou M, et al. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci (2010). Link
- [4] Kimura KD, et al. The C. elegans thermosensory neuron AFD responds to warming. Curr Biol (2004) Pubmed link
- [5] Ramot D, et al. Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci (2008). Link
- [6] Beverly M et al. Degeneracy and Neuromodulation among Thermosensory Neurons Contribute to Robust Thermosensory Behaviors in Caenorhabditis elegans. J Neurosci (2011). Link
- [7] Li J, Huang K, Le W. Establishing a novel C. elegans model to investigate the role of autophagy in amyotrophic lateral sclerosis. Acta Pharmacol Sin (2013). Pubmed link
- [8] Bahrami AK, Zhang Y. When females produce sperm: genetics of C. elegans hermaphrodite reproductive choice. G3 (Bethesda) Pubmed link
- [9] Corsi AK, Wightman B, Chalfie M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics (2015).
- [10] BIOL3530: Molecular and Developmental Biology, Development of Nematodes, Sea Urchins, and Ascidians. Link
- [11] Researchers track neuron branching’s genetic control switch | Research News @ Vanderbilt | Vanderbilt University. Link
- [12] Emmons SW. Male development. WormBook (2005) Link
- [13] Karen Oegema lab | Ludwig Cancer Research
- [14] Davies T. et al. Using fast-acting temperature-sensitive mutants to study cell division in Caenorhabditis elegans. (2017) Link
FAQ
A temperature-sensitive mutant is a powerful tool used in C. elegans biology. These are worms with a specific mutation, often engineered, that causes a protein to be functional at one "permissive" temperature but non-functional or inactivated at a different "sensitive" or "restrictive" temperature. This characteristic allows researchers to precisely control the activation or inactivation of a specific gene by simply shifting the temperature. This method is used to question the role of a protein, and its absence, during processes like development, cell division, or neuron formation.
C. elegans possess a sophisticated system for sensing temperature, which is part of a process called thermotaxis. Several types of sensory neurons are involved. The AFD neurons are central to this process. They use a cGMP-dependent network of molecules to regulate an ion channel. Within this network, temperature itself regulates the concentration of cGMP in the cytoplasm. The level of cGMP is what determines how many cGMP-gated ion channels are open at a specific temperature. Other neurons, like the ASI chemosensory neurons, are also related to thermotaxis under very specific conditions, and their response is dependent on the AFD neurons.
C. elegans is used as a model organism to study several human neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, ALS, and Huntington’s diseases. In the case of ALS and Huntington’s, it has been shown that temperature-sensitive mutant genes can act as modifiers for processes central to these diseases. Specifically, they can modify protein misfolding and the toxicity associated with protein aggregation. This allows researchers to use temperature shifts to control these pathogenic processes in a living animal model.
While temperature control is useful, there are important constraints to consider.
Sterility: If C. elegans* populations are grown at temperatures more than 25°C, the nematodes can become sterile. Since the worms are mostly hermaphrodites with the capacity to self-fertilize, this loss of reproductive capacity is a major issue. This makes accurate temperature control necessary when studying the worm’s physiology. * Male Production: The heat shock technique, while common for analyzing behavior, can also be a limitation. Too much exposure time to high temperatures (always over 25°C) can increase the production of males in the population. To avoid this bias, it is important to tightly control the timing of the temperature exposure.